Abstract
Aims
Left bundle branch block (LBBB) is the most common conduction disorder after transcatheter aortic valve replacement (TAVR) with an increased risk of atrioventricular (AV) block. The aim of the current study was to identify non-invasive predictors for infranodal conduction delay in patients with LBBB.
Methods
We analyzed consecutive patients undergoing TAVR with pre-existing or new-onset LBBB between August 2014 and August 2020. His ventricular (HV) interval measurement was performed on day 1 after TAVR. Baseline, procedural, as well as surface and intracardiac electrocardiographic parameters were included. Infranodal conduction delay was defined as HV interval > 55 ms.
Results
Of 825 patients screened after TAVR, 151 patients (82 ± 6 years, 39% male) with LBBB were included. Among these, infranodal conduction delay was observed in 25%. ΔPR (difference in PR interval after and before TAVR), PR and QRS duration after TAVR were significantly longer in the group with HV prolongation. In a multivariate analysis in patients with sinus rhythm (n = 123), ΔPR (OR per 10 ms increase: 1.52; 95%CI: 1.19–2.01; p = 0.002) was the only independent factor associated with infranodal conduction delay. A change in PR interval by 20 ms yielded a specificity of 83% and a sensitivity of 46%, with a negative predictive value of 84% and a positive predictive value of 45% to predict HV prolongation.
Conclusions
Simple analysis of surface ECG and a calculated ΔPR < 20 ms can be used as predictor for the absence of infranodal conduction delay in post-TAVR patients with LBBB.
Graphical abstract

Similar content being viewed by others
Introduction
Transcatheter aortic valve replacement (TAVR) has become a well-established treatment option for patients with symptomatic aortic stenosis with an intermediate-to-high surgical risk [1,2,3,4]. Periprocedural complications of TAVR have decreased with improved operator experience as well as newer transcatheter heart valve technologies [5, 6]. However, conduction disturbances, in general, and left bundle branch block (LBBB), in particular, remain a common problem after TAVR, due to the anatomical proximity of the conduction system to the aortic valve [7]. Despite the relatively high incidence of LBBB ranging between 4 and 65% [6], its management is still ambiguous. It is known that patients with new-onset LBBB after TAVR have an increased risk of all-cause mortality, cardiovascular mortality, rehospitalization and progression to complete atrioventricular block [5, 8, 9]. Simplified algorithms for the treatment of conduction disturbance after TAVR were proposed based on non-invasive electrocardiographic (ECG) measurements in a recent expert panel document [1, 10]. For selective patients with pre-existing conduction disturbance as well as new-onset LBBB, an invasive electrophysiological study (EPS) may be conducted to assess the risk of high-degree atrioventricular conduction block (HAVB) based on infranodal conduction and consequently guide the decision for permanent pacemaker implantation (PPI) [10,11,12]. Recently, an EPS-tailored strategy was proposed to stratify patients with LBBB after TAVR regarding the development of HAVB based on a His ventricular (HV) interval cutoff of > 55 ms [13].
The objective of this study was to identify clinical and ECG predictors of infranodal conduction disturbance (defined as HV interval > 55 ms) in patients with LBBB after TAVR. This may aid clinical decision making in patients with LBBB after TAVR.
Methods
Study design and patient population
We analyzed the data from patients collected in the prospective Swiss TAVR registry (NCT01368250) for the period from August 2014 to August 2020, treated at our institution. Written informed consent was obtained from all patients and the study was approved by the local ethics committee.
Patients included in the present analysis were those with pre-existing or new-onset LBBB after TAVR. The clinical and procedural characteristics were obtained from the electronic patient medical records. Exclusion criteria were TAVR performed through other than transfemoral implantation route, valve-in-valve implantation, previous PPI, and HAVB after TAVR requiring PPI, and missing electrophysiological (EP) measurement of the HV interval. Valve types included in our study were self‐expandable Evolut R and Evolut R Pro (Medtronic, Minneapolis, MN), Portico (St. Jude Medical, St Paul, MN), Acurate NEO (Boston Scientific, Natick, MA), balloon‐expandable Sapien 3 (Edwards Life Science, Irvine, CA), or mechanically expandable Lotus and Lotus Edge (Boston Scientific Inc., Marlborough, MA).
Transcatheter aortic valve replacement
TAVR procedures were performed as previously described [13]. Briefly, transthoracic echocardiography, coronary angiography, and ECG-triggered multislice computed tomography scan of the aorta were performed for procedural planning. The implantation of the valve was performed according to the recommendations of the manufacturer. During the procedure, a temporary pacemaker using a quadripolar catheter (5Fr, CRD, St Jude Medical, USA) was positioned in the right ventricular apex. After implantation, patients were transferred to the intensive care unit overnight with the temporary pacemaker left in place and programmed to VVI 30 bpm in case of HAVB. Continuous rhythm monitoring by telemetry was performed for 72 h.
Electrocardiographic assessment
The 12-lead electrocardiograms obtained with a standard ECG recorder (Schiller, Baar, Switzerland) prior to and during hospital stay were analyzed. Each ECG recording was assessed for rhythm and conduction disturbance with a sweep speed of 25 mm/s and standard augmentation of 1 mV/10 mm. First-degree atrioventricular block was defined as a PR interval ≥ 200 ms. LBBB was defined using conventional criteria with a QRS duration ≥ 120 ms, an R wave peak delay in lead V5/V6 of > 60 ms, and an rS or QS in lead V1 and V2 [14]. The automatically calculated PR interval and QRS duration by the ECG recorder were included in the analysis. To improve the accuracy of the measurements, each ECG was manually reviewed, and if necessary, corrected.
We defined the difference of the PR interval and the QRS duration before and after the procedure as ΔPR and ΔQRS, respectively. To be able to use PR interval for prediction models, only patients with sinus rhythm were included. Patients with atrial fibrillation were included in a second analysis.
Electrophysiology study after TAVR
In all patients showing LBBB on the ECG the day after the procedure, we performed a limited EPS [13]. In brief, intracardiac measurements were obtained using the quadripolar diagnostic catheter (5F, CRD, St. Jude Medical) used as a temporary pacemaker wire during TAVR. After withdrawal of the catheter from the ventricle to the His position, HV interval was measured over three consecutive beats using the electronic calipers with a sweep speed of 100 mm/s on the EP system (Sensis, Siemens, Germany). Simultaneously, PR and QRS durations were obtained. Based on these measurements, patients were stratified into a group with normal HV interval (HV ≤ 55 ms) and with prolonged HV interval (HV > 55 ms).
Follow-up
Follow-up was performed 3 months after TAVI, including physical examination, 12-lead ECG and transthoracic echocardiography. In patients with pacemaker implantation due to prolonged HV interval, pacemaker interrogation was performed. No need for pacing was defined as < 1% ventricular pacing and intrinsic 1:1 AV conduction with the device programmed to VVI 30 bpm [13].
Statistical analysis
Continuous variables are presented as mean and standard deviation or median (interquartile range) and categorical variables as numbers and percentages. T test was used for continuous, normally distributed and the Wilcoxon test for skewed variables. Categorical variables were compared using Chi-square or the Fisher’s exact test as appropriate.
Using logistic regression models, we first performed a univariate analysis to identify unadjusted associations between baseline data, procedural characteristics, ECG parameters and the HV prolongation > 55 ms. Potential predictors with a p value ≤ 0.05 were selected for multivariable analysis and subsequently corrected for patient age, sex and body surface area.
Receiver-operating characteristic (ROC) curves were generated and the area under the curve (AUC) was calculated for uni- and multivariate analyses. The optimal thresholds (cutoffs) to predict a prolonged HV interval (HV > 55 ms) were determined based on the Youden’s index [15] as well as for an easy and clinically applicable cutoff of 10 ms and 20 ms. All statistical analyses were performed using R version 4.0.2 (R Foundation for statistical computing, Vienna, Austria).
Results
Baseline data
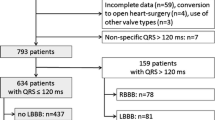
After exclusion of 332 of the 825 patients based on the described criteria, 181 of the 493 (37%) patients remaining showed LBBB the day after the procedure (Fig. 1). After exclusion of patients declining EPS (n = 22) and patients with intermittent LBBB (n = 8), the final cohort available for analysis consisted of 151 patients. The mean age was 82 ± 6 years, 39% of patients were male (Table 1). The median gradient across the aortic valve was 48 (IQR 39; 59) mmHg, the mean aortic valve area was 0.7 ± 0.2 cm2. On EPS 1 day after TAVR, a prolonged HV interval was detected in 25% of patients and a normal HV interval was detected in 75% of patients.
The demographic and pre-procedural echocardiographic parameters were comparable between patients with normal and prolonged HV interval except an increased body surface area (1.92 ± 0.31 versus 1.82 ± 0.22; p = 0.041) and a higher prevalence of diagnosis of atrial fibrillation (55 versus 34%; p = 0.030) in the group with a prolonged HV interval. No differences between the two groups were observed for the valve types implanted. Exclusion of patients with pre-existing LBBB (n = 22) showed comparable results (Supplement 1). The median PR interval and QRS duration before TAVR were normal in both groups. ECG parameters before and after TAVR are listed in Table 2.
Follow-up data
After exclusion of 7 of the 38 patients with prolonged HV interval (3 patients rejected PPI, 1 death before follow-up due to non-cardiac causes, and 3 patients without device interrogation showing pacing percentages), 19 of the 31 patients (61%) showed a need for pacing ≥ 1%. Of the 113 patients without HV prolongation and LBBB after TAVR, four (4%) showed an indication for PPI based on a documented episode of HAVB (three of four from implantable loop recorders (ILR)). During follow-up, however, none of the patients with ILR documented HAVB showed need for pacing (0%) within 3 months of follow-up.
Predictors of prolonged HV interval
In the 123 patients with sinus rhythm during EPS, ΔPR values (odds ratio (OR) per 10 ms increase: 1.49; 95% confidence interval (CI): 1.19–1.93; p = 0.001), the PR interval (OR per 10 ms increase: 1.27; 95% CI: 1.11–1.48; p = 0.001) and QRS duration after TAVR (OR per 10 ms increase: 1.37; 95% CI: 1.03–1.84; p = 0.031) were significantly associated with prolonged HV interval. After correction for age, sex and body surface area in the multivariate analysis, ΔPR (OR per 10 ms increase: 1.52; 95% CI: 1.19–2.01; p = 0.002) was the only parameter associated with prolonged HV interval (Table 3). Additional correction for hypertension and a clinical diagnosis of AF (but with sinus rhythm before and after TAVR) did not result in relevant changes of our model (OR per 10 ms increase of ΔPR 1.41; 95% CI: 1.09–1.88; p = 0.013) (Supplement 2).
When including patients with atrial fibrillation on the ECG before and after TAVR in the analysis (n = 151), the QRS width post-TAVR (OR per 10 ms increase: 1.46; 95% CI: 1.13–1.93; p = 0.006), but not the ΔQRS (OR per 10 ms increase: 0.95–1.32; p = 0.196) was identified as a predictor of HV prolongation. After correction for sex, age, body surface area and class III antiarrhythmic therapy, QRS post-TAVR (OR: 1.34; 95% CI: 1.02–1.79; p = 0.041) remained a significant predictor in the multivariate analysis (Supplement 3).
The AUC for the ROC curve for ΔPR to predict a prolonged HV was 0.724 (95% CI: 0.610–0.837) in the univariate and 0.763 (95% CI: 0.653–0.873) in the multivariate analysis corrected for age, sex and body surface area, respectively (Fig. 2).
With a cutoff of ≥ 10 ms or ≥ 16 ms for ΔPR chosen based on the criteria of a clinical applicable cutoff and the Youden’s index, the sensitivity and specificity to predict HV prolongation was 79% and 62% and 61% and 79%, respectively. The positive (PPV) and negative predictive value (NPV) was 38% and 91% for a ΔPR of ≥ 10 ms or 46 and 87% for a ΔPR of ≥ 16 ms, respectively. The recently recommended clinically relevant ΔPR value of ≥ 20 ms [1] yielded a sensitivity of 46% and specificity of 83% with a PPV of 45% and NPV of 84% to predict HV prolongation.
Discussion
The main findings of our study are: (1) prolongation of infranodal conduction delay (HV interval > 55 ms) in patients with LBBB after TAVR is frequent (25%). (2) PR interval, QRS duration and ΔPR were significantly longer after TAVR in the group with prolonged HV interval compared to the group with normal HV interval. (3) ΔPR was identified as the only independent predictor for HV prolongation with an OR 1.49 (95% CI 1.19–1.93) per increase of 10 ms in the univariate analysis. (4) The AUC of the ROC curve was 0.724 (95% CI) for ΔPR. (5) Finally, the absence of a relevant increase in PR interval (< 20 ms) rules out a critical HV interval prolongation (> 55 ms) with an NPV of 84%.
Conduction disturbances and specifically LBBB are the most common complications after TAVR [16]. LBBB has been shown to be associated with a high rate of complete AV block (20%) and syncope (16%) during the first year after TAVR compared to < 1% in patients without LBBB [8]. Due to the lack of clear guidelines, individual treatment strategies are currently implemented. A recent expert consensus decision document states that EPS (and PPI) should be considered in patients with new, progressive, or pre-existing conduction disturbance changing after the procedure [10]. In addition, in patients with persistent new-onset LBBB the day after TAVR, an invasive EPS to guide the decision for PPI was proposed in case of defined ECG changes (PR interval > 240 ms or QRS duration > 150 ms) by a scientific expert panel [1]. In pre-existing LBBB, it is recommended to consider EPS in case additional ECG changes after TAVR are observed, defined as further increase (> 20 ms) in QRS duration or PR interval, a QRS duration > 150 ms, or a PR interval > 240 ms [1]. However, evidence for these proposed cutoffs of the QRS duration and PR interval is scarce in LBBB patients after TAVR and their value to predict HAVB is unclear. The presence of an infranodal conduction delay, defined as a prolonged HV interval, can be measured in an EPS and has been shown to correlate with the development of HAVB. However, since EPS after TAVR is not readily available in all centers, the identification of non-invasive predictors for prolonged HV interval based surface ECG parameters (PR interval and QRS duration) is of high clinical relevance.
Value of HV measurements to predict HAVB
The first description of the impact of the HV interval measurements on the prevalence of HAVB in patients with chronic LBBB was reported almost three decades ago [17]. They observed that an HV interval ≥ 70 ms was an independent predictor for progression to HAVB in patients with LBBB. More recently, several studies implemented a predefined HV cutoff after TAVR to trigger for PPI in general. The value for the HV interval range between 55 ms [12, 13], over 65 ms [18], 70 ms [19], 75 ms [11, 20], 80 ms [21], up to 100 ms [22]. Whether prophylactic PPI in the setting of prolonged HV interval improves outcomes (e.g., HAVB, syncope, hospitalizations, mortality) has not been assessed these studies and warrants further investigation in a randomized study.
Rivard et al. [18] showed a strong association of the post-procedural HV interval with HAVB. In their study, an HV interval ≥ 65 ms predicted HAVB with 83.3% sensitivity and 81.6% specificity and 82% NPV and 62% PPV. Similarly, we showed with an HV interval cutoff > 55 ms a 67% sensitivity, 84% specificity, and 90% NPV and 53% PPV [13]. In a smaller study by Mirolo et al. [19], three of five patients (60%) with PPI due to new-onset LBBB and an HV interval > 70 ms had significant ventricular pacing during follow-up of 2–4 months, reflecting episodes of HAVB. This need for pacing was similar to our observations with an HV cutoff of 55 ms.
In general, the timing of EPS may play an important factor, since the conduction behavior after TAVR might change, especially within the first 24 h [10]. Furthermore, the behavior may be different for self-expandable compared to balloon-expandable valves [23]. In this study, EPS was performed the day after TAVR and it is unclear whether EPS immediately after TAVR would yield similar results.
Since EPS is not universally available, the relationship between the intracardiac measurements (HV interval) and easily accessible clinical and surface ECG parameters is of high clinical importance. Readily available parameters that could replace the HV interval measurement may simplify clinical decision making. In our analysis, we identified the ΔPR interval as an indicator for increased risk of HV prolongation. This suggests that a simple analysis of the dynamic changes in the ECG post-TAVR could identify patients at risk for developing HAVB. This observation is in line with a previous study analyzing the occurrence of delayed AVB (> 48 h) after TAVR. [24] Significant widening of the QRS and PR interval in patients within 48 h after TAVR was observed; however, only ΔPR proved to be an independent predictor for delayed AVB in a multivariate analysis. At the other end of the spectrum, and potentially more relevant for clinical practice and streamlining workflow, in our study, the absence of a relevant increase in PR interval (< 20 ms) was able to make HV interval prolongation unlikely with a negative predictive value of 84%.
Study limitations
This was a single-center retrospective study focusing on LBBB after TAVR. Other conduction disturbances such as RBBB and fascicular block in conjunction with RBBB were not studied. Furthermore, numerous valve types were included. However, when analyzing subgroups of self-expandable (CoreValve, Evolut R, Evolut Pro, Portico, Acurate NEO, n = 97), balloon-expandable (Sapien 3, n = 20) and mechanical-expandable valves (Lotus, Lotus Edge; n = 34), ΔPR was still identified as a significant predictor for HV prolongation (Supplement 4). The predictor ΔPR was only available for patients in sinus rhythm after TAVR because PR interval cannot be determined in AF. This problem could be circumvented by cardioversion, but this is not performed in clinical routine. However, when including the 20 patients in AF (13%) and consequently without PR interval as covariate, ΔQRS was the only significant predictor of a prolonged HV interval in a univariate analysis, but not after correction for other confounders in a multivariate analysis. Therefore, invasive EPS for HV interval assessment might play a more important role in patients with AF on ECG before and after TAVR. Finally, interobserver variability can be significant with ECG interpretation.
Conclusion
Simple surface ECG criteria can be used to identify patients with LBBB at risk of an increased HV interval after TAVR. A prolongation of the PR interval of more than 20 ms was identified as independent predictor for a prolonged HV interval > 55 ms. In contrast, the absence of a significant increase in the PR interval (ΔPR < 20 ms) makes significant HV interval prolongation > 55 ms unlikely with a negative predictive value of 84%.
Abbreviations
- ECG:
-
Electrocardiographic
- EPS:
-
Electrophysiological study
- TAVR:
-
Transcatheter aortic valve replacement
- HAVB:
-
High-degree atrioventricular conduction block
- HV:
-
His ventricular
- LBBB:
-
Left bundle branch block
- PPI:
-
Permanent pacemaker implantation
References
Rodés-Cabau J, Ellenbogen KA, Krahn AD et al (2019) Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC Scientific Expert Panel. J Am Coll Cardiol 74:1086–1106
Leon MB, Smith CR, Mack MJ et al (2016) Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374:1609–1620
Leon MB, Smith CR, Mack M et al (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363:1597–1607
Reiter C, Lambert T, Kellermair J et al (2021) Intraprocedural dynamics of cardiac conduction during transcatheter aortic valve implantation—assessment by simultaneous electrophysiology testing. Hear Rhythm 8:419–425
Nazif TM, Chen S, George I et al (2019) New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: an analysis from the PARTNER II trial. Eur Heart J 40:2218–2227
Auffret V, Puri R, Urena M et al (2017) Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation 136:1049–1069
Judson GL, Agrawal H, Mahadevan VS (2019) Conduction system abnormalities after transcatheter aortic valve replacement: mechanism, prediction, and management. Interv Cardiol Clin 8:403–409
Urena M, Mok M, Serra V et al (2012) Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve. J Am Coll Cardiol 60:1743–1752
Regueiro A, Abdul-Jawad Altisent O, Del Trigo M et al (2016) Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Interv 9:e003635
Lilly SM, Deshmukh AJ, Epstein AE et al (2020) 2020 ACC expert consensus decision pathway on management of conduction disturbances in patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol 76:2391–2411
Tovia-Brodie O, Ben-Haim Y, Joffe E et al (2017) The value of electrophysiologic study in decision-making regarding the need for pacemaker implantation after TAVI. J Interv Card Electrophysiol 48:121–130
Makki N, Dollery J, Jones D, Crestanello J, Lilly S (2017) Conduction disturbances after TAVR: electrophysiological studies and pacemaker dependency. Cardiovasc Revascularization Med 18:S10–S13
Knecht S, Schaer B, Reichlin T et al (2020) Electrophysiology testing to stratify patients with left bundle branch block after transcatheter aortic valve implantation. J Am Heart Assoc 9:e014446
Jastrzębski M, Kukla P, Kisiel R, Fijorek K, Moskal P, Czarnecka D (2018) Comparison of four LBBB definitions for predicting mortality in patients receiving cardiac resynchronization therapy. Ann Noninvasive Electrocardiol 23:e12563
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Lin SI, Miura M, Tagliari AP et al (2020) Intraventricular conduction disturbances after transcatheter aortic valve implantation. Interv Cardiol 15:e11
Scheinman MM, Peters RW, Suavé MJ et al (1982) Value of the H-Q interval in patients with bundle branch block and the role of prophylactic permanent pacing. Am J Cardio 50:1316–1322
Rivard L, Schram G, Asgar A et al (2015) Electrocardiographic and electrophysiological predictors of atrioventricular block after transcatheter aortic valve replacement. Heart Rhythm 12:321–329
Mirolo A, Viart G, Durand E et al (2018) Pacemaker memory in post-TAVI patients: who should benefit from permanent pacemaker implantation? PACE Pacing Clin Electrophysiol 41:1178–1184
Akin I, Kische S, Paranskaya L et al (2012) Predictive factors for pacemaker requirement after transcatheter aortic valve implantation. BMC Cardiovasc Disord 12:87
Badenco N, Chong-Nguyen C, Maupain C et al (2017) Respective role of surface electrocardiogram and His bundle recordings to assess the risk of atrioventricular block after transcatheter aortic valve replacement. Int J Cardiol 236:216–220
Rogers T, Devraj M, Thomaides A et al (2018) Utility of invasive electrophysiology studies in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Am J Cardiol 121:1351–1357
Coeman M, Kayaert P, Philipsen T et al (2020) Different dynamics of new-onset electrocardiographic changes after balloon- and self-expandable transcatheter aortic valve replacement: implications for prolonged heart rhythm monitoring. J Electrocardiol 59:68–73
Mangieri A, Lanzillo G, Bertoldi L et al (2018) Predictors of advanced conduction disturbances requiring a late (≥48 H) permanent pacemaker following transcatheter aortic valve replacement. JACC Cardiovasc Interv 11:1519–1526
Funding
Open Access funding provided by Universität Basel (Universitätsbibliothek Basel). None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Christian Sticherling: reports grants from the European Union’s FP7 program, Biosense-Webster and lecture and consulting fees from Abbott, Medtronic, Biosense-Webster, Boston Scientific, Microport, and Biotronik all outside the submitted work. Michael Kühne: reports grants from the Swiss National Science Foundation, Swiss Heart Foundation, Bayer, Pfizer BMS, Boston Scientific, personal fees from Bayer, Böhringer Ingelheim, Pfizer BMS, Daiichi Sankyo, Medtronic, Biotronik, Boston Scientific, Johnson & Johnson all outside the submitted work. Sven Knecht: reports grant from the Freiwillige Akademische Gesellschaft (FAG) Basel and the Stiftung Kardiovaskuläre Forschung. Others: none.
Ethics approval
This study was ethics approved and performed in accordance with the Declaration of Helsinki.
Consent to participate
All patients provided written informed consent to participate in the study.
Consent for publication
All authors read and consented to the final manuscript version for publication.
Additional information
Sven Knecht and Michael Kühne: shared last authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Auberson, C., Badertscher, P., Madaffari, A. et al. Non-invasive predictors for infranodal conduction delay in patients with left bundle branch block after TAVR. Clin Res Cardiol 110, 1967–1976 (2021). https://doi.org/10.1007/s00392-021-01924-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01924-w