Abstract
Background: Severe functional tricuspid regurgitation is associated with adverse clinical outcomes. Conventional surgical risk for patients with advanced tricuspid regurgitation stage is high. Alternative approaches are therefore required.
Aims: The purpose of this study was to investigate the feasibility, technical details, and midterm outcomes of implantation of a novel radial force-independent orthotopic transcatheter tricuspid valve –the LuX-Valve– in patients with severe functional tricuspid regurgitation.
Methods: The implantations of the LuX-Valve were performed in six patients with left-sided valvular surgery and secondary massive tricuspid regurgitation. The anatomy of the tricuspid valve and right heart measurements were evaluated by echocardiography and computed tomography in all patients. Conventional right ventricular (RV) function parameters and RV global longitudinal strain were obtained at baseline and 12-month follow-up. LuX-Valve implantation was performed under the guidance of digital subtraction angiography and transoesophageal echocardiography.
Results: All patients had successful implantations of LuX-Valves through the right atrium with a substantial reduction in the degree of tricuspid regurgitation. Although one patient with moderate paravalvular leakage died because of right heart failure during three-month follow-up, the five patients who lived had no significant paravalvular leakage, and displayed significant improvements in mean transvalvular gradient, right heart sizes, conventional RV function indices and RV global longitudinal strain, and a reduction in New York Heart Association Functional Class during 12-month follow-up.
Conclusions: Transcatheter tricuspid valve replacement with the LuX-Valve was feasible and safe for patients with severe functional tricuspid regurgitation. This strategy seems a promising treatment option for patients at high surgical risk.
Introduction
Functional tricuspid regurgitation (TR) is a common condition in patients with left-sided heart valve disease. If left untreated, TR can progress and lead to right ventricular (RV) failure. Conventional surgery is limited due to the relatively higher risk of morbidity and mortality1,2,3,4. As a consequence, alternative approaches are required. Percutaneous intervention for valvular heart disease is one of the fastest growing fields in interventional cardiology. Transcatheter valve procedures for aortic, mitral, and pulmonary valve disease have been increasingly applied in clinical practice5,6. Recently, as therapeutic alternatives for high-risk patients, transcatheter treatments of TR have been emerging7,8. Unlike the case of aortic and mitral valves, the anatomic features of the tricuspid annulus make percutaneous technologies for the treatment of TR challenging, owing to the lack of stable adjacent structures for the device to be securely anchored. A previous study reported that transcatheter tricuspid devices, which were employed with the radial force between the device and tricuspid annulus, were radial force-dependent7,8. However, this radial force for valve fixation may cause complications, such as conduction block and right coronary artery impingement9,10. Here, we report a single-site series of patients who underwent transcatheter tricuspid valve (TV) replacement with the novel radial force-independent LuX-Valve (Jenscare Biotechnology Co. Ltd., Ningbo, China).
Methods
PATIENTS
Six patients with left-sided valvular surgery and secondary massive TR were consecutively recruited from Wuhan Union Hospital, China, between June 2019 and September 2019 for transcatheter tricuspid valve replacement with the LuX-Valve. All patients were deemed to be at high or extreme risk for a conventional approach and were thoroughly discussed by our Heart Team before they underwent this procedure. Informed consent for the procedure was obtained from all patients, who were instructed about the potential risks and benefits related to this intervention.
Inclusion criteria included age ≥50 years, severe TR, New York Heart Association (NYHA) functional class ≥III and left ventricular ejection fraction (LVEF) ≥50%. Exclusion criteria included LVEF <50%, pulmonary artery systolic pressure ≥60 mmHg (by right heart catheterisation), tricuspid annular plane systolic excursion (TAPSE) <10 mm, RV fractional area change (RV FAC) <20%, untreated severe coronary artery disease, Ebstein’s anomaly, congenital RV dysplasia, and RV outflow obstruction.
DEVICE DESCRIPTION
The LuX-Valve system is composed of a stent bioprosthesis and a delivery system (Figure 1). The valve stent consists of four components: 1) a trileaflet prosthetic valve with treated bovine pericardium; 2) a self-expanding nitinol valve stent consisting of an atrial disc; 3) a bird tongue-shaped interventricular septal anchoring component; and 4) two expanded polytetrafluoroethylene-covered graspers. The delivery system consists mainly of the inner sheath, outer sheath and core. The diameter of the delivery system is 32 Fr. The outer sheath is flexible and can be bent up to 45 degrees. The stent bioprosthesis is available in four sizes (30 to 55 mm) and eight skirt-shaped disc models intended for native tissue tricuspid annular diameters of 25 to 50 mm (Supplementary Table 1).
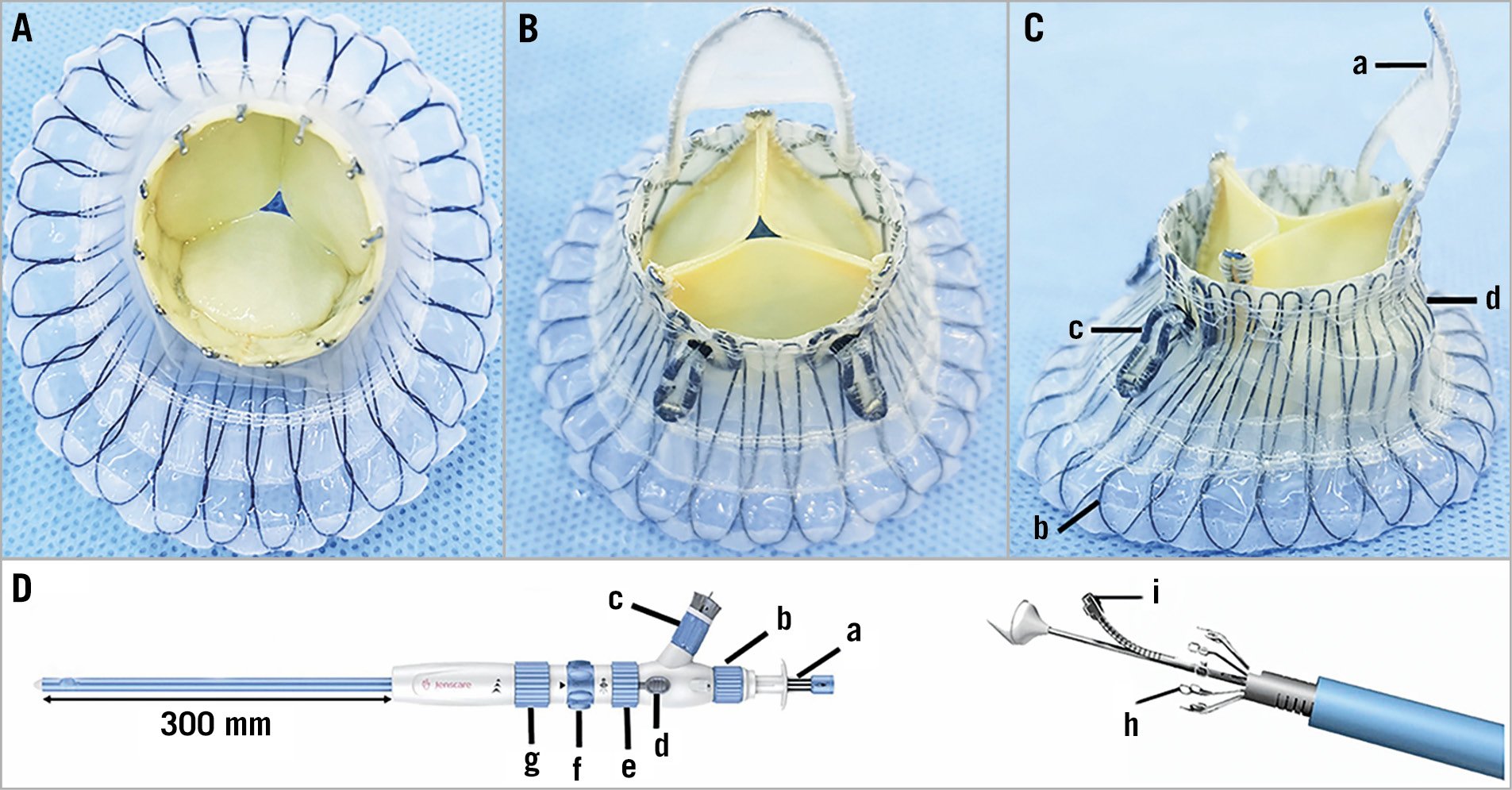
Figure 1. Views of the LuX-Valve. A) Right atrium view. B) Right ventricle view. C) The LuX-Valve device includes a bird tongue-shaped interventricular septal anchoring component (a), a self-expanding nitinol valve stent consisting of an atrial disc (b), two expanded polytetrafluoroethylene-covered graspers (c), and a trileaflet prosthetic valve with treated bovine pericardium (d). D) The delivery system includes a needle control (a), a needle-containing tube bending knob (b), a needle separation control element (c), a locker (d), a valve release knob (e), an outer sheath bending knob (f), an outer sheath release knob (g), an anchoring needle (h), and a valve grasper (i).
TRANSTHORACIC ECHOCARDIOGRAPHY
Transthoracic echocardiography (TTE) examinations were performed using an Epiq7C ultrasound platform with a 1 to 5 MHz convex probe (Philips Medical Systems, Andover, MA, USA) before surgery and one year after intervention. Left ventricular and RV measurements including left atrial end-systolic volume index (LAESVI), left ventricular end-diastolic volume index (LVEDVI), right atrial end-systolic volume index (RAESVI), RV end-diastolic area index (RVEDAI), LVEF, TAPSE, and RV FAC were performed according to the American Society of Echocardiography guidelines11. Speckle tracking analysis was performed using TomTec Image-Arena software (TOMTEC Corp., Chicago, IL, USA) for assessment of RV global longitudinal strain, using the apical four-chamber view. Abnormal cut-off values for RV function parameters were as follows: TAPSE <17 mm, RV FAC <35%, RV global longitudinal strain >–20%11. Tricuspid annular (TA) diameter and TR severity were determined in the apical four-chamber view. Quantitation of the degree of TR was performed using multiple methods according to a previous publication including effective regurgitant orifice area (EROA) and vena contracta width12. Pulmonary artery pressure was estimated using right heart catheterisation. Pulmonary hypertension was defined as mean pulmonary artery pressure greater than 20 mmHg.
TRANSOESOPHAGEAL ECHOCARDIOGRAPHY
Comprehensive transoesophageal echocardiography (TEE) examinations were performed using an Epiq7C ultrasound platform with a X8-2t probe (Philips Medical Systems) before and after the stent valve device implantation. Focused TV imaging was performed using both two-dimensional (2D) and three-dimensional (3D) modalities. The minimum and maximum diameters of the tricuspid annulus were measured in end-diastole using 3D volumes. TEE was performed to evaluate the position of the bioprosthesis and the central or paravalvular TR after the bioprosthesis implantation.
COMPUTED TOMOGRAPHY
Computed tomography angiography (CTA) was performed with two imaging systems (Aquilion ONE; Canon Medical Systems, Otawara, Japan, and SOMATOM Force; Siemens, Forchheim, Germany). Imaging was acquired at heart rates between 60 and 90 beats/min after the administration of beta-blocker therapy if required. Imaging was performed with retrospective electrocardiographic (ECG) gating and whole-heart coverage single beat acquisition. CT covered the cardiac area and reconstructed every 10% from 0-90% of the R-R interval. Intravenous injection of contrast agent (iodixanol) was performed using a triphase protocol as follows: pure contrast at a rate of 4-6 mL/s, followed by 20-30 mL saline flush.
Image data were reconstructed and analysed using Mimics Medical 20.0/3-Maticmedical 20.0 post-processing software. Attachment points of the TV leaflet were determined by multiplanar reformation (MRP) imaging tool. The TA saddle shape can be clearly displayed in the 3D space. The highest point of the saddle shape was located at the intersection of the anterior valve and the septal valve (Figure 2).
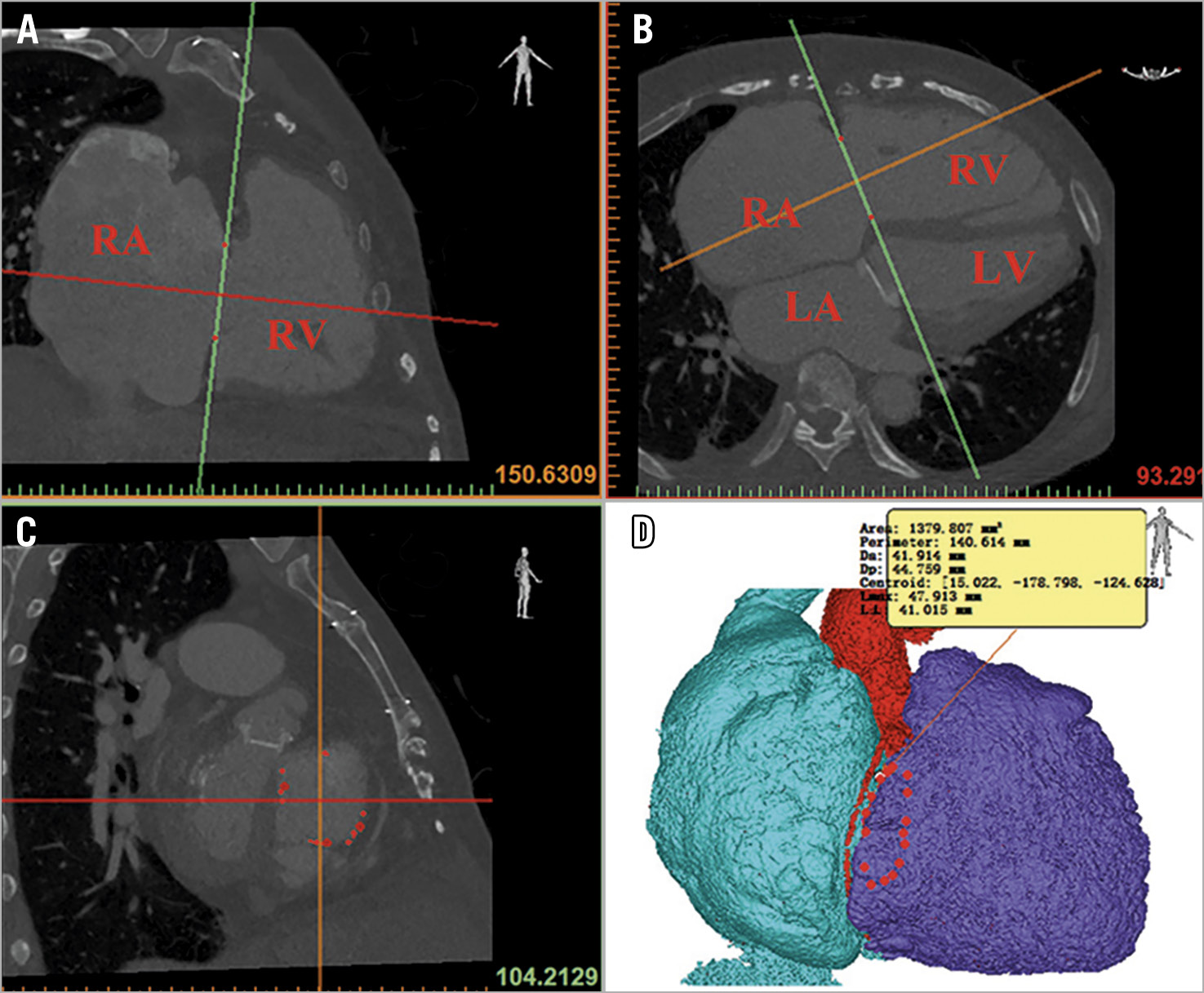
Figure 2. Tricuspid annulus assessment using CTA. Attachment points of the tricuspid valve leaflet were determined using multiplanar reformation imaging (A-C). The minimum and maximum diameters of the tricuspid annulus were measured by a planar cross-sectional area (D). LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle
The TA plane was determined by the highest point of the saddle shape and two points corresponding to the lowest area of the annulus. The minimum and maximum diameters of the tricuspid annulus were measured by a planar cross-sectional area. The best transatrial access was the intersection of the right atrium perpendicular to the midpoint of the tricuspid annulus.
PROCEDURAL STEPS
The procedure was performed via a right anterior lateral thoracotomy in the intercostal space identified by preoperative CT (Figure 3A, Figure 3B). The delivery system was inserted into the right atrium through a purse string sutured in the middle of the exterior surface of the right atrium. Under the guidance of TEE and digital subtraction angiography (DSA) (Figure 3C, Central illustration), the delivery system was centred in the annulus perpendicular to the annular plane by adjusting the angle of the sheath. After the delivery sheath was confirmed as being placed into the right ventricle, the outer sheath was gradually drawn back and the anchoring component was first released, followed by the stent valve and anterior leaflet graspers. When the graspers were completely released, the operator gently pulled back the entire delivery system attempting to hook the anterior TV leaflet, which was accomplished when the resistance force was similar to a fish biting on the end of a fishing line. At this moment, the graspers could be observed to swing with the movement of the TV by X-ray and TEE, indicating that the graspers had grasped the anterior leaflet. Then, the atrial disc was released, and the device could still be finely adjusted to minimise paravalvular leakage under the guidance of TEE. When the orientation and position of the valve were satisfactory, the anchoring needle was pushed forward until it penetrated through the anchoring component and was nailed into the ventricular septum. Eventually, the stent valve device was firmly anchored.
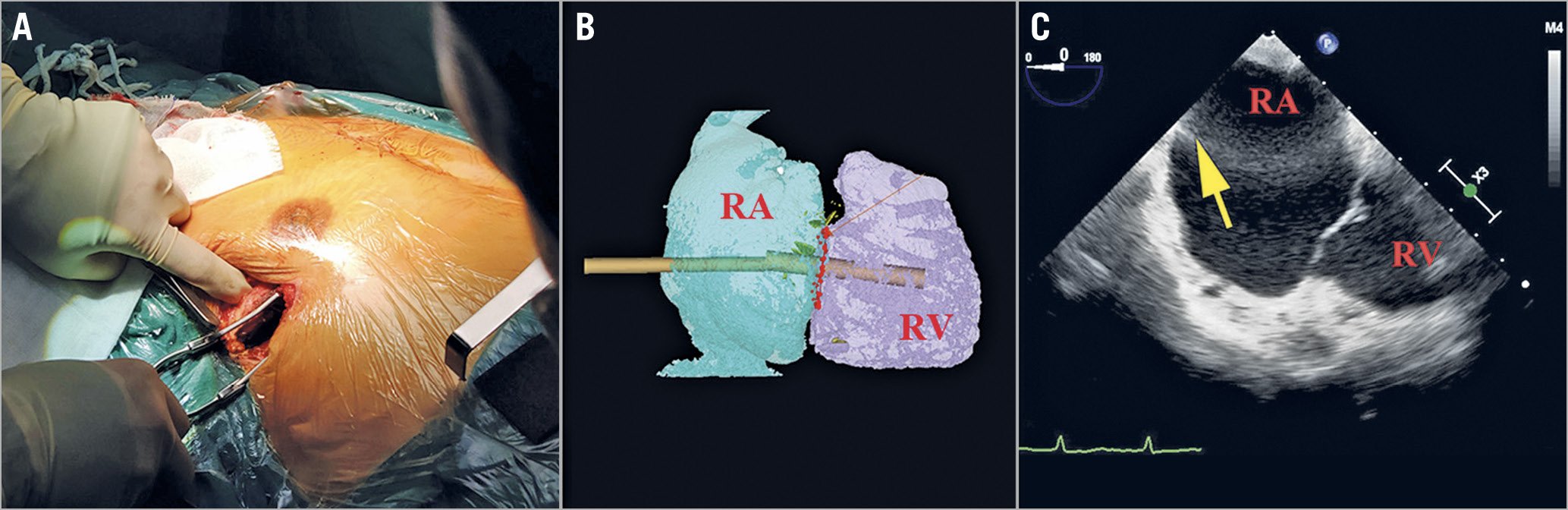
Figure 3. Path planning for the delivery system. A minimally invasive right anterior lateral thoracotomy was performed in the intercostal space (A) using a transatrial access approach, in which the most co-axial deployment angle to the TA was determined by 3D reconstruction of this location (B). Balloting the right atrial (RA) wall with a forceps (yellow arrow) was performed to confirm the location of the atriotomy and intended trajectory for delivery of the atrioventricular valved stent (C). RA: right atrium; RV: right ventricle
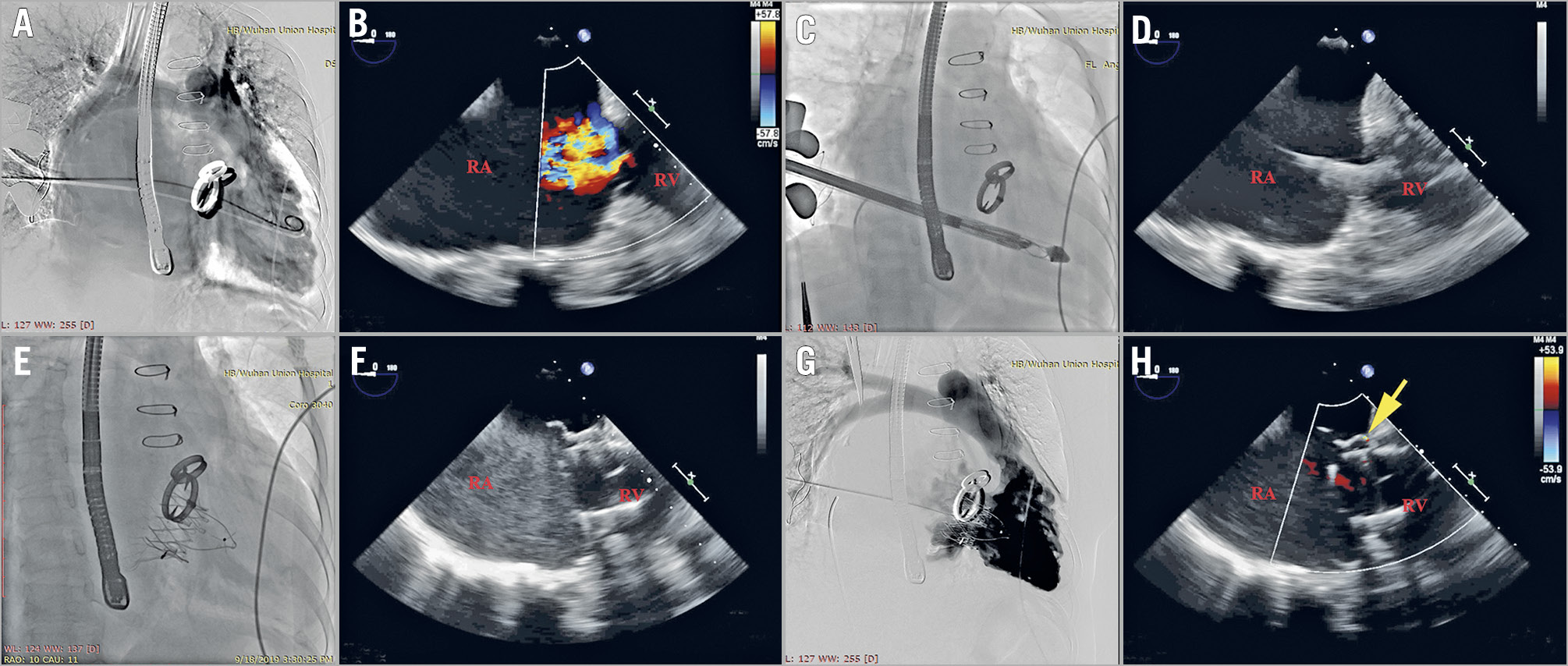
Central illustration. LuX-Valve implantation under the guidance of TEE and digital subtraction angiography (DSA). Tricuspid regurgitation was noted on DSA and colour Doppler echocardiography before surgery (A & B). Under the guidance of TEE and DSA, we adjusted the angle of the sheath so that the delivery system was centred in the annulus perpendicular to the annular plane (C & D). The atrial disc was released, and the device could still be finely adjusted to minimise paravalvular leakage until the orientation and position of the device were satisfactory, which was guided by the echocardiography (E & F). Paravalvular regurgitation (yellow arrow) was evaluated by DSA and colour Doppler echocardiography after bioprosthesis implantation (G & H). RA: right atrium, RV: right ventricle
Results
CLINICAL CHARACTERISTICS
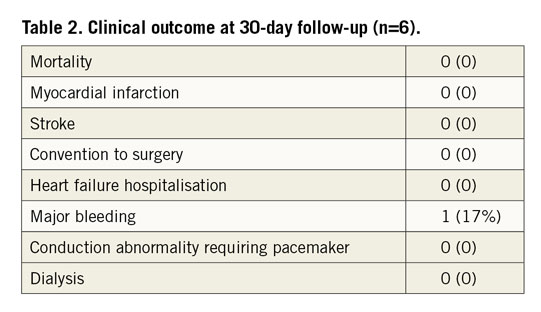
A total of 6 patients (6 women; 56 [53 to 60] years) were enrolled in the study. Of these subjects, 3 were in NYHA Functional Class IV and 3 in NYHA Functional Class III. The clinical characteristics of these patients are listed in Table 1. Four patients (66.6%) had atrial fibrillation, one had ventricular premature beat, and one had hepatitis and intestinal tuberculosis. All patients had left-sided valvular surgery, including four patients with mitral valve replacement, and two with mitral and aortic valve replacement. Five patients presented with pulmonary hypertension. Technical success was 100% without intraprocedural mortality or other complications during 30-day follow-up (Table 2). The average device release time was 9.7 (6.2 to 13.6) minutes. The median length of postoperative stay in the intensive care unit was 3.5 days (interquartile [IQ] range: 2 to 7 days). Although one patient experienced significant bleeding of the chest wall during the operation, there was no need for conversion to open surgery. There was no stroke or myocardial infarction. No patients needed a temporary or permanent pacemaker. The median length of postoperative hospitalisation stay was 8 days (IQ: 6 to 11 days). All discharged patients were on anticoagulants. By one-year follow-up, the patient (#1) with moderate paravalvular TR had died three months postoperatively due to non-improvement of right heart failure; all other patients reported an improvement in symptoms. All patients who remained alive were in NYHA Functional Class I or II.
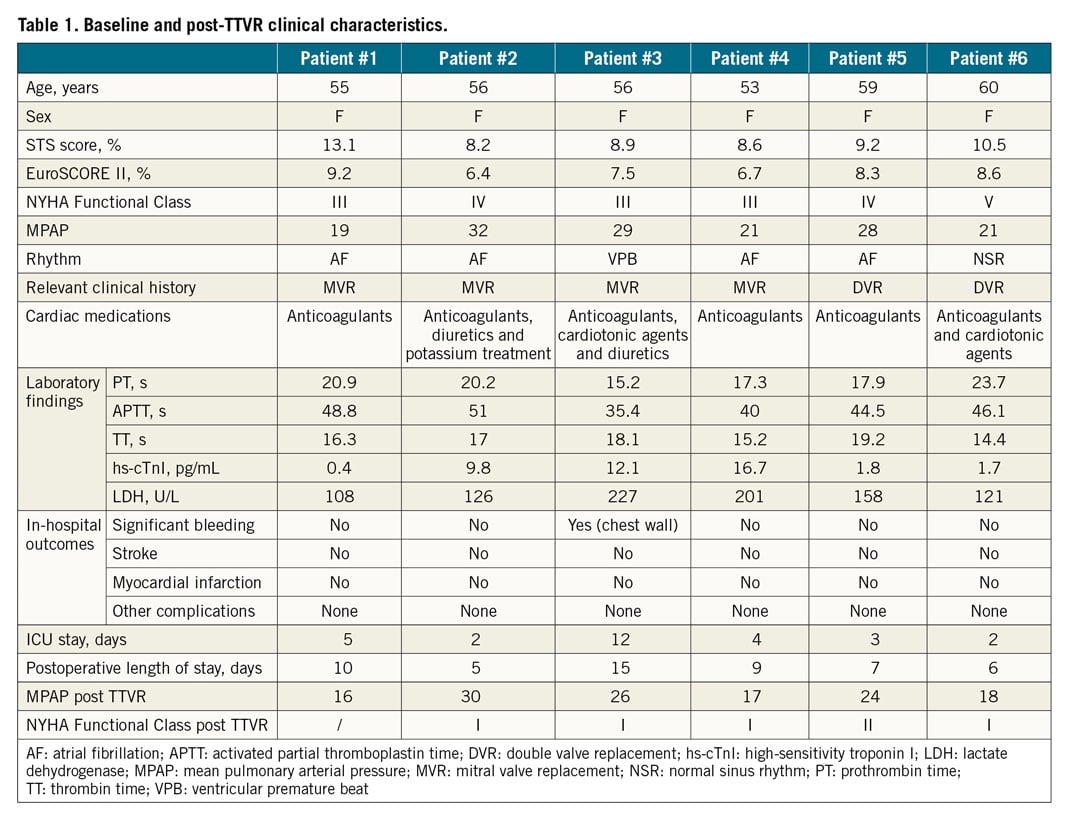
BASELINE AND FOLLOW-UP ECHOCARDIOGRAPHY
Baseline and follow-up echocardiographic data are listed in Table 3. Right heart dilation was noted in all cases. A reduced TAPSE was found in four patients, and a decreased RVFAC was noted in three patients. While RV global longitudinal strain, a more sensitive index of RV dysfunction, was significantly decreased in all patients. TA diameters measured by 2D and 3D echocardiography were increased in all patients, and slightly smaller than those in CT measurements. All patients had left atrial enlargement to some extent, which may be related to the history of left heart valve disease. No patient had left ventricular dysfunction. Colour Doppler echocardiography showed that all patients had severe TR. Post-device TEE revealed moderate paravalvular regurgitation in 1 patient (#1), mild paravalvular regurgitation in 3 patients (Supplementary Figure 1A, Supplementary Figure 1B), and no paravalvular regurgitation in 2 patients.
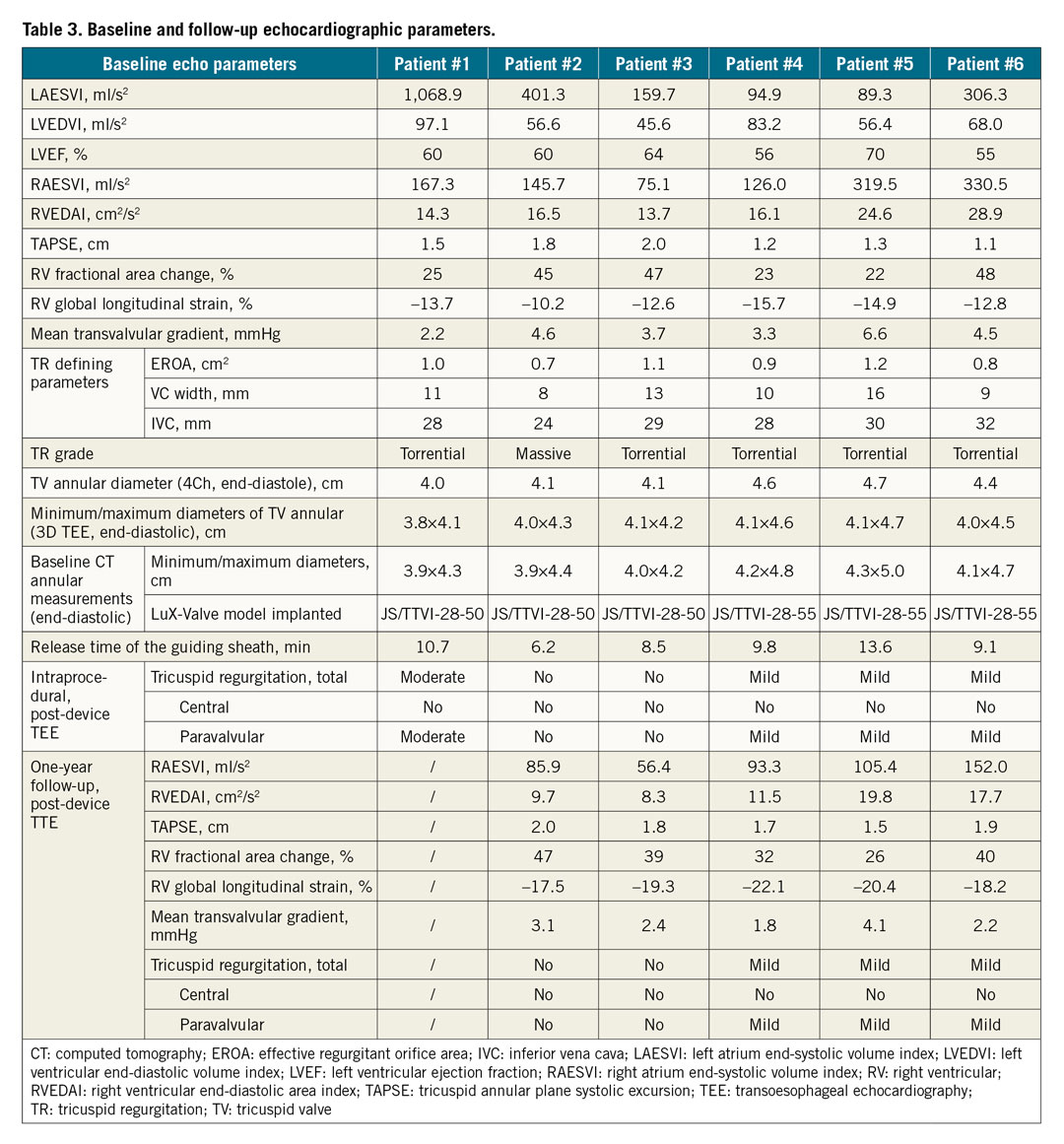
Although one patient (patient #1) died at three months after LuX-Valve implantation because of the continuous existence of RV failure, the other five patients were doing well and had minimal symptoms during 12-month follow-up. At 12-month follow-up, TTE suggested that the LuX-Valve was still functioning well for the five patients alive. Compared with baseline data, the mean transvalvular gradient, RAESVI, RVEDAI and RV global longitudinal strain improved, while TAPSE and RV FAC showed no significant difference. All five living patients, especially those three patients with mild paravalvular TR, had no change in the severity of paravalvular regurgitation at one-year follow-up. Additionally, valve thrombosis or leaflet thickening by echo/CT was not noted in these patients.
Discussion
Our results showed that all patients with severe functional TR were successfully implanted with the LuX-Valve. After implantation, TR severity improved significantly, in spite of one patient with moderate perivalvular leakage and three patients with mild perivalvular leakage. At 12-month follow-up, this small series of patients showed improvements in symptoms and physical capacity from NYHA Functional Class IV/III to NYHA I or II except for one patient who died due to RV failure. In addition, the paravalvular leak did not progress for those three patients with mild perivalvular leakage.
Surgical treatment of severe functional TR has been recommended to improve prognosis13. However, traditional or redo TV surgery in patients with severe functional TR is associated with high mortality1,2,14,15. In this study, six patients with severe functional TR had left heart valve replacement and symptoms of right heart failure, which suggested that open redo surgery for those patients had to be rejected due to high mortality risk. For those patients, new percutaneous transcatheter approaches could offer a treatment alternative to address this unmet clinical need, such as transcatheter tricuspid valve repair (TTVr) or transcatheter tricuspid valve replacement (TTVR). Since it has been reported that most repair devices resulted in incomplete reduction in TR, TTVR should be the better option.
In recent years, Navia et al reported the first case of successful TTVR at the native TA level using the NaviGate bioprosthesis device (NaviGate Cardiac Structures, Inc., Lake Forrest, CA, USA)16. After that, several recent studies have demonstrated that the bioprosthesis relied on radial force to secure the tricuspid annulus8,16,17. However, radial forces make it difficult to immobilise the prosthetic valve due to several anatomic peculiarities of the tricuspid annulus, such as the larger tricuspid annulus, the coaxiality of the delivery system and the potential for direct impingement on important anatomic structures surrounding the tricuspid annulus, and may cause complications9,10. In the present study, a novel radial force-independent TTVR device, namely the LuX-Valve, was applied at the TA level via the right atrium access in six patients with severe TR secondary to left heart disease. The implant was successful in all patients, with a short release time of the guiding sheath, as mentioned earlier. In addition, device malposition or high transvalvular gradients were not identified by echocardiography during follow-up, which suggested that the LuX-Valve was feasible. Why was the LuX-Valve so reliable? The secure fixation of the Lux-Valve at the native TA level was based on the following methods: intraventricular anchoring with a septal needle insertion, anterior leaflet grasping with two clips, and atrial side support with an atrial disc. For these reasons, the LuX-Valve can be implanted for severe TR patients with a larger TA diameter without displacement.
A single-centre Chinese study reported 12 cases of LuX-Valve implantation; the majority of these patients had left-sided valvular surgery and/or coronary artery bypass grafting. The mean transvalvular gradient decreased significantly, and the degree of valve regurgitation improved in all but one patient. One patient experienced vasospastic myocardial infarction and died during hospitalisation; the mean follow-up period was 30 days18. Although the study showed that the LuX-Valve can be implanted successfully and safely, with good short-term outcomes in most patients, there is relatively limited information about midterm or long-term outcomes after LuX-Valve implantation. Here, we evaluated midterm (12 months) outcomes in a small cohort of patients who underwent LuX-Valve implantation. Although one patient died three months after intervention due to the continuous existence of RV failure, the major therapeutic effect of this procedure for the other five patients appeared to be a reduction in TR without significant valve regurgitation or perivalvular leakage, and with a normal TV inflow gradient. At 12-month follow-up, echocardiography revealed that the LuX-Valve was functioning well. RV size and global longitudinal strain improved significantly. Device malposition, significant perivalvular leakage or valve thrombosis was not observed in our study. Furthermore, there was no evidence of embolisation of prosthetic valves into right cardiac chambers or the pulmonary artery, or other complications.
Limitations
There were several limitations in this study. This was a small initial series of patients, and the findings should be considered preliminary. More robust estimates will be required in a larger patient population. A limitation of this device is the lack of radial force for annular sealing that reduces conduction block but increases paravalvular leak. Although the feasibility of transfemoral TTVR has been reported9, the delivery system of the LuX-Valve could not be used percutaneously through the peripheral vein in this present study due to the complicated structure of this device. Compared with the less invasive percutaneous transfemoral/transjugular catheterisation approach, the LuX-Valve was implanted via mini-thoractomy with a surgical approach, which carried a higher risk in these patients. Recently, the second generation of the LuX-Valve, with a transjugular delivery system, has been developed. However, it is undergoing animal tests and will be applied in clinical practice in the future.
Conclusions
In conclusion, TTVR with the LuX-Valve has been proved to be feasible and safe in our case series of patients with severe functional TR. Interventional reduction of TR was associated with improved clinical symptoms for right heart failure and without major complications. Despite our very promising midterm results, further larger controlled trials and long-term outcomes are necessary to improve the level of evidence and the quality of results for tricuspid LuX-Valve implantation.
|
Impact on daily practice Surgical treatment of severe functional TR has been demonstrated to have high mortality, suggesting that traditional open heart TV surgery may be inadequate for certain patients. TTVR could offer a treatment alternative to address this unmet clinical need. This study shows that TTVR with the LuX-Valve is feasible and safe for patients with severe functional TR. |
Acknowledgements
We gratefully acknowledge colleagues in the Department of Cardiac Surgery and the Department of Radiology for providing beautiful DSA and CT pictures during this study.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81701716; 81727805; 81922033) and the National Natural Science Foundation of Hubei (Grant No. 2018CFB568).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.




