Abstract
This expert opinion paper on cardiac imaging after acute ischemic stroke or transient ischemic attack (TIA) includes a statement of the “Heart and Brain” consortium of the German Cardiac Society and the German Stroke Society. The Stroke Unit-Commission of the German Stroke Society and the German Atrial Fibrillation NETwork (AFNET) endorsed this paper. Cardiac imaging is a key component of etiological work-up after stroke. Enhanced echocardiographic tools, constantly improving cardiac computer tomography (CT) as well as cardiac magnetic resonance imaging (MRI) offer comprehensive non- or less-invasive cardiac evaluation at the expense of increased costs and/or radiation exposure. Certain imaging findings usually lead to a change in medical secondary stroke prevention or may influence medical treatment. However, there is no proof from a randomized controlled trial (RCT) that the choice of the imaging method influences the prognosis of stroke patients. Summarizing present knowledge, the German Heart and Brain consortium proposes an interdisciplinary, staged standard diagnostic scheme for the detection of risk factors of cardio-embolic stroke. This expert opinion paper aims to give practical advice to physicians who are involved in stroke care. In line with the nature of an expert opinion paper, labeling of classes of recommendations is not provided, since many statements are based on expert opinion, reported case series, and clinical experience.
Similar content being viewed by others
Introduction
Whereas imaging of the brain and the brain-supplying arteries as well as electrocardiogram (ECG) monitoring is standard in stroke diagnostics [1, 2], discussion of the clinical impact and appropriate method of cardiac imaging is ongoing [3, 4]. As the available evidence is limited, present guideline recommendations on cardiac imaging after ischemic stroke or TIA remain vague and in principle, the indication of cardiac imaging remained at the discretion of the treating physician. As an example, the European Stroke Organization (ESO) guideline issued in 2008 recommends echocardiography in selected stroke patients, e.g. in case of suspected cardioembolism (Class III, Level B recommendation) [5]. However, no recommendations were given as to the choice of transthoracic (TTE) or transoesophageal (TOE) echocardiography. In a recent consensus statement from the ESO-Karolinska Stroke Update Conference, TTE was considered the primary choice for cardiac imaging (Grade A), while TOE and bubble test-transcranial Doppler were recommended in patients with an embolic stroke of undetermined source (ESUS) for PFO detection (Grade A) as well as TOE over TTE to detect aortic atheroma (Grade C) [6]. Recently updated guidelines on acute stroke management consider echocardiography reasonable in selected ischemic stroke patients to guide secondary stroke prevention (American Heart Association, Class IIa recommendation, expert opinion) and to determine whether eligibility criteria for PFO closure are met (American Heart Association, Class IIa recommendation) or in case of cryptogenic stroke (German Society of Neurology & German Cardiac Society, Class Ia recommendation) [1, 7]. The American Society of Echocardiography guidelines were published in 2016 and recommend routine use of TTE as a screening tool for potential cardiac sources of embolism, while TOE might be considered as an initial or supplemental test in specific cases, e.g. suspicion for endocarditis [8]. Cardiac CT and MRI should be reserved to selected patients with high suspicion for cardioembolism and inconclusive results after echocardiography. Due to its semi-invasive nature, TOE is not recommended if potential results will not change therapeutic decisions [8]. The Canadian Stroke Best Practice Recommendations for Acute Stroke Management, issued in 2018, recommended considering echocardiography in cases where a stroke mechanism has not been identified (Evidence Level C) or a cardiac cause of stroke is suspected, in patients with suspected embolic stroke and normal neurovascular imaging (Level B) and no contraindications for anticoagulant therapy [9].
About one out of five strokes is cardio-embolic in nature and stroke-recurrence rates are comparably high after cardio-embolic stroke. A cardiac source of embolism is more likely if multiple acute or subacute strokes in different vascular territories are detected, secondary hemorrhagic transformation is observed or a Valsalva maneuver preceded symptom onset [10]. There are several reasons why cardiac imaging should be performed in patients after ischemic stroke or TIA. First, most stroke patients are cardiovascular high-risk patients and the likelihood of alterations in cardiac structure and/or function is comparably high. Second, cardiac imaging helps to identify the most probable cause of ischemic stroke or TIA and subsequently lead to changes in secondary stroke prevention [11,12,13]. Third, imaging findings may guide further diagnostic management in stroke patients, e.g. intensified search for atrial fibrillation (AF) [14,15,16]. Fourth, certain imaging findings may have immediate therapeutic implications, e.g. endocarditis [17, 18].
This expert opinion paper collates and weighs the prevalence of stroke-related cardiac pathologies and most commonly used cardiac imaging methods with a focus on left atrial imaging. Summarizing present knowledge, expert-based suggestions for practical application of cardiac imaging after ischemic stroke or TIA are provided. Using the Delphi method, experts answered questionnaires in two rounds. Given (key point) recommendations are labeled as follows: ** if all experts agreed; * if the vast majority (≥ 90%) of experts agreed; without * if the majority (≥ 60–89%) of experts agreed.
Prevalence of cardiac abnormalities in stroke patients
Cardiac and aortic sources of cerebral embolism resulting in stroke/TIA are heterogeneous [19]. A potential cardiac source of stroke can be identified in about 30% of unselected ischemic stroke patients [20]. Cardiac pathologies with embolic risk appear to have a similar frequency in older and younger stroke patients [21, 22]. A systematic review summarizing the results of very heterogeneous studies on the prevalence of cardiac findings showed that the most frequent findings in stroke patients are patent foramen ovale (PFO) and atrial septal aneurysm (ASA) [4]. While a PFO is prevalent in about one-quarter of all humans, PFO prevalence is higher in younger stroke patients with otherwise cryptogenic stroke (44–54% in case series) [4, 23,24,25,26]. An ASA is detected in 4–20% of all stroke patients and is accompanied by a PFO in about 60% [23, 26, 27]. Left atrial (LA) appendage (LAA) thrombus is a rather rare finding in stroke patients with an estimated prevalence of about 3% according to recent meta-analyses with even lower detection rate in stroke patients presenting in sinus rhythm [28,29,30]. Spontaneous echo contrast in the left atrium is observed in a broad range from 2 to 15.5% of stroke patients. Its etiological relevance is poorly understood, and its visualization depends on the echocardiographic equipment, sedation of the patients, and machine settings, which may help explain the large heterogeneity [31, 32]. Whereas mitral valve abnormalities were reported in about 10% of all stroke patients, rheumatic heart disease was less common in Europe [4]. A significantly reduced left ventricular ejection fraction was found in 2–16.7% of all stroke patients [4, 33, 34]. An empiric classification predominantly based on old echocardiographic studies divides cardiac conditions into high risk or low/unknown embolic risk (Fig. 1) [35, 36]. Less common potential sources of embolism include degenerative mitral valve stenosis, cardiomyopathies including myocarditis and storage disorders (e.g. amyloidosis, Fabry disease), atrial and ventricular communications, aortic abnormalities (e.g. related to Libman Sacks), and vasculitis (e.g. Kawasaki disease).
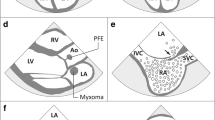
Scheme of the different topographies of the ultrasound probes and example imaging planes to illustrate differences in the ability to visualize cardiac structures, modified from [35, 36]. Common potential sources of embolism (high risk—blue, minor/unclear—green) or indicators of cardiovascular disease (black) are shown. Further, less common potential sources of embolism and echocardiographic findings are provided in Table 1
Treatment strategy changes by the initiation of anticoagulation have been reported in 3.4–8.0% [37, 38].
Cardiac imaging after ischemic stroke
Echocardiography
The most widely used imaging technology in post-stroke patients is echocardiography, which can be performed by TTE or TOE. TOE usually has a higher accuracy for the detection of potential cardio-embolic sources. Due to the anatomical neighborhood of heart and esophagus the TOE probe comes close to the heart permitting use of high-frequency ultrasound probes (4.2–7.4 MHz) enabling a high spatial and temporal image resolution [22, 39]. The combination of both bedside procedures permits a comprehensive evaluation of cardiac structure and function in real-time (Fig. 1).
TOE is a safe examination if rare contraindications are respected [40]. In many patients, TOE is well tolerated with pharyngeal and esophageal local anesthesia. A mild sedation is often used for a more convenient introduction of the TOE probe into the distal esophagus and the stomach. However, the patient should still be able to cooperate, e.g. perform Valsalva maneuver for PFO detection. Close hemodynamic monitoring is required if sedatives are used [39]. TOE is well-suited to examine valvular abnormalities (e.g. caused by endocarditis) and perpendicular imaging planes as well as multidimensional imaging help to assess valve function despite artifacts produced by mechanical heart valves that are frequent in TTE examinations. Furthermore, TOE is the gold standard technique for imaging of the morphology and structure of the atria including the interatrial septum, and the LAA, which is usually not visualized by TTE [22, 41]. Sometimes, the diagnosis of a PFO and atrial communication defects can be made by TTE if sufficient quality images can be generated.
TTE is non-invasive and well suited for the assessment of the morphology and function of cardiac cavities, especially the left ventricle and left atrium, as well as for the assessment of hemodynamically significant valvular pathologies. Contrast-enhanced TTE with ultrasound agent opacification of the cavities of the left heart can improve endocardial delineation, exclude left ventricular thrombi and support ischemia diagnosis during stress echocardiography. It should be performed in patients with poor acoustic windows or the suspicion of a left ventricular thrombus. TTE is limited by impaired ultrasound penetrance, especially in obese individuals or patients with pulmonary disease.
Key points
-
Echocardiography is feasible and safe in patients with (acute) ischemic stroke or TIA.**
-
TTE and TOE have to be considered as complimentary methods that provide distinct aspects of cardiac pathologies.**
-
TOE is the method of choice for refined imaging of the atria and the interatrial septum. Details of (peri-)valvular abnormalities can be diagnosed with higher accuracy.**
-
TTE is able to assess global and regional wall motion, ventricular abnormalities and left atrial function.**
Systematic imaging and documentation of a comprehensive, standardized echocardiography work-up is illustrated in Fig. 2 (TTE), Fig. 3 (TOE) and Supplementary Table 1.
Image acquisition transthoracic echocardiography protocol in post-stroke patients. Parasternal long axis view for measurements of left ventricular (LV) diameters, left ventricular outflow tract (LVOT) diameter, LV-wall thickness, dimensions of the aortic arch (a); parasternal short axis view at the level of the mitral valve (MV) for assessment of MV pathologies, detection or exclusion of MV stenosis or MV regurgitation (b); parasternal short axis view at the level of the aortic valve (AV) for assessment of AV pathologies, detection or exclusion of AV stenosis or AV regurgitation (c); parasternal short axis view at the level of the pulmonary valve (PV) and pulsed wave (pw) Doppler spectrum of the right ventricular (RV) outflow tract (RVOT) flow to assess RV stroke volume for pulmonary stroke volume (Qp)/ systemic stroke volume (Qs)—calculation (d); parasternal short axis view of the interatrial septum to detect or exclude atrial communication defects (e); apical long axis view for assessment of LV function using deformation imaging (f); color-coded apical long axis view for assessment of AV function including pw Doppler spectrum of the LVOT flow to assess LV stroke volume for Qp/Qs-calculation (g), if AV is pathological a continuous wave (cw) Doppler spectrum has to be added; color-coded apical long axis view for assessment of MV function including pw Doppler spectrum of the transmitral flow (h), if MV is pathological a cw Doppler spectrum has to be added; apical 2-chamber view for assessment of LV function using deformation imaging (i); apical 4-chamber view for assessment of LV function using deformation imaging (j); color-coded apical 4-chamber view for assessment of tricuspid valve function including cw Doppler spectrum to assess systolic pulmonary artery pressure (sPAP) (k); color-coded apical 4-chamber view of the interatrial septum to detect or exclude atrial communication defects (l); color-coded tissue Doppler apical 4-chamber view including tissue pw Doppler spectrum of the basal septal myocardial velocities (m); color-coded tissue Doppler apical 4-chamber view including tissue pw Doppler spectrum of the lateral septal myocardial velocities (n); subcostal view of the inferior caval vein to document systemic volume state (o); subcostal short axis view of the interatrial septum to detect or exclude atrial communication defects (p); subcostal short axis view at the level of the pulmonary valve and pw Doppler spectrum of the RVOT flow to assess RV stroke volume for Qp/Qs-calculation, if parasternal view is not possible (q); suprasternal view of the aortic arch to detect or exclude aortic dissection and other aortic pathologies (r). AV aortic valve, cw continuous wave, LV left ventricular, LVOT left ventricular outflow tract, MV mitral valve, pw pulsed wave, Qs systemic stroke volume, Qp pulmonary stroke volume, RV right ventricular, RVOT right ventricular outflow tract
Image acquisition transoesophageal echocardiography protocol in post-stroke patients. Midoesophageal 4-chamber view for assessment of LV function using deformation imaging, if documentation using transthoracic echocardiography (TTE) is not possible (a); midoesophageal 2-chamber view for assessment of LV function using deformation imaging, if TTE documentation is not possible (b); midoesophageal long-axis view for assessment of LV function using deformation imaging, if TTE documentation is not possible (c); conventional 2D-documentation at least in 2 different sectional planes of the interatrial septum (IAS) (d); color-coded 2D-documentation at least in 2 different sectional planes of the IAS to document interatrial communication defects (e); contrast 2D-documentation with agitated saline at least in 2 different sectional planes of the IAS to document patent foramen ovale (PFO). Ideally no or mild sedation of the patient is performed and the Valsalva maneuver practiced with the patient before administration of agitated saline contrast agent. If available, 3D transoesophageal probes should be used and the test repeated several times, if negative. Good documentation is necessary to distinguish inter-atrial from trans-pulmonary shunts (f); contrast 3D-documentation with agitated saline to document PFO, if possible (g); conventional 2D-documentation at least in 2 different sectional planes of the left atrial appendage (LAA) (h); color-coded 2D-documentation at least in 2 different sectional planes of the LAA to exclude or document LAA thrombus formation (i); pulsed wave (pw) Doppler spectrum of the LAA flow velocities (j); 3D-documentation of the LAA, if possible (k); conventional 2D-documentation of the long axis and short axis view of the aortic valve (AV) (l); color-coded 2D-documentation of the long axis and short axis view of the AV (m); 3D-documentation of the AV and the aortic root complex, if possible (n); color-coded 3D-documentation of the AV and the aortic root complex, if relevant AS or AR is present and if possible (o); conventional 2D-documentation of the long axis and short axis view of the mitral valve (MV) (p); color-coded 2D-documentation of the long axis and short axis view of the MV (q); 3D-documentation of the MV, if possible (r); color-coded 3D-documentation of the MV, if relevant MS or MR is present and if possible (s); conventional 2D-documentation of the descending aorta/aortic arch (t); 3D-documentation of the descending aorta/aortic arch, if possible (u). AR aortic valve regurgitation, AS aortic valve stenosis, AV aortic valve, IAS interatrial septum, LAA left atrial appendage, LV left ventricular, MR mitral valve regurgitation, MS mitral valve stenosis, MV mitral valve, PFO patent foramen ovale, pw pulsed wave, TTE transthoracic echocardiography
With the rapid advancement of imaging methods with increasingly high resolution and short image acquisition duration, other modalities such as cardiac CT/CT angiography as well as cardiac MRI have become available for diagnostics after stroke. A semi-quantitative comparison of the different imaging modalities and specific indications is provided in Table 1. Cardiac CT carries the burden of a comparatively high radiation dose despite a number of dose reduction strategies that have been introduced over the last years [42,43,44]. Cardiac MRI comes at higher costs and requires comparatively long image acquisition times [45].
Cardiac computed tomography
ECG-gated cardiac scanning with multi-detector 64-slice CT systems that scan large volumes at high speed (short breath hold) the temporal and spatial resolution significantly improves and enables visualization of small and volatile structures [46]. The use of ECG-gated imaging protocols has led to a significant decrease in the radiation dose for cardiac CT with a pooled radiation dose of 3.5 mSv according to a meta-analysis from 2013 [43, 44] and German cardiac CT registry experience [47]. Contrast-enhanced cardiac CT can produce high-quality images of the cardiac walls and lumen, the coronary arteries and the large vessels including the aortic arch and descending aorta. In a meta-analysis, LA and LAA thrombi were detected with a sensitivity of 96% and a negative predictive value of 99% compared to the gold standard TOE, which permits rule-out [48]. Its specificity for thrombi is limited by pseudo-filling defects due to blood stasis, which can be optimized by using two-phase or dual-enhanced computed tomography image acquisition, i.e. including late-phase images [49]. Thus, in a meta-analysis from 2013 cardiac CT exhibited reliable diagnostic characteristics for the detection of atrial clots, when delayed imaging is performed [48]. In addition, dual-energy cardiac CT acquiring images at different energy levels may improve the detection of LAA thrombus by differentiating clots from slow blood flow, obviating the need for delayed imaging and thus additional radiation exposure [50].
Furthermore, cardiac CT can contribute to the differentiation of left ventricular thrombi and other intraventricular masses due to its higher spatial resolution and improved delineation of endocardial borders, but CT usually follows initial evidence from an echocardiogram in the clinical routine [51, 52]. The latter remains the standard for follow-up examinations. CT has the advantage of virtually unrestricted imaging views with isotropic spatial resolution [51, 52]. On the other hand, cardiac CT generates limited information on tissue characteristics compared to MRI and relies on anatomical features to suggest the tumor entity. The diagnostic accuracy for PFO and ASA is limited because small, mobile structures are not well delineated and PFO diagnosis usually requires Valsalva maneuver [53]. In patients with suspected or proven endocarditis, CT imaging can reveal life-threatening perivalvular complications such as abscess or mycotic aneurysms, which is of particular importance in patients with prosthetic heart valves, increasingly transcatheter heart valve replacement [54]. Functional imaging by positron emission tomography (PET) combined with CT uses higher metabolic activity in inflammatory tissue for the diagnosis of endocarditis [55]. So far, no direct comparisons for TOE versus CT or PET-CT for the diagnosis of endocarditis and its complications are available. CT may also support the diagnosis and quantification of the extent of valvular calcifications as potential sources of embolism [56]. In addition, CT angiography or calcium scoring are clinically established for the diagnostic classification and risk stratification of patients with suspected or known coronary artery disease (CAD) [57, 58] However, little data exist on its diagnostic or prognostic value in post-stroke patients [59].In addition, modern methods including CT-derived functional flow reserve and CT perfusion may help to also evaluate ischemia [60]. In stroke patients, cardiac CT is a feasible alternative, but diagnostic accuracy compared to TOE is limited as outlined above [53, 61,62,63,64].
Key points
-
Cardiac CT is feasible in stroke patients due to the short acquisition time; however, it comes at the cost of radiation exposure.*
-
Cardiac CT can be a complementary imaging method for additional work-up of specific patients with acute ischemic stroke or TIA, but is not optimal for the evaluation of most frequent cardiac sources of embolism.*
Cardiac MRI
Cardiac MRI is emerging as a non-invasive tomographic imaging method for post-stroke cardiac work-up [45, 65]. Today, cardiac MRI requires comparatively long image acquisition times and affords the patient to follow breathing commands, limiting the feasibility in stroke patients. This may be overcome by modern non-breath-hold and compressed sequences available for CINE and late gadolinium enhancement (LGE) imaging. MRI has become the gold standard for ventricular volumes and mass. It can visualize complex cardiac abnormalities and quantify hemodynamics including valve disease and permits excellent soft tissue characterization. Stress MRI can assess cardiac ischemia and LGE uses cellular integrity to show myocardial viability, fibrosis, and scarring. It may be superior to echocardiography in the diagnosis of previous (clinically silent) myocardial infarction. Delayed-enhancement cardiac MRI is the gold standard for left ventricular thrombus detection due to its excellent tissue delineation with an absence of contrast enrichment in thrombus material [66, 67].
After initial TTE, cardiac MRI can further aid to differentiate unclear, less common cardiomyopathies, e.g. in case of cardiac amyloidosis or non-compaction cardiomyopathy [68,69,70]. In a recent two-center study, cardiac MRI detected seven cases of prior unknown cardiomyopathy in 132 patients with ischemic stroke and no cardiac source of embolism on TTE [71]. While the detection rate of LA thrombi correlates well with TOE, the diagnosis of LAA structures, evaluation of the interatrial septum and PFO has remained technically challenging and is not reliable yet [45, 72].
The feasibility of cardiac MRI in consecutive stroke patients has been demonstrated in a single-center study as 89 of 103 patients with acute ischemic stroke completed the 50-min examination [45]. The use of cardiac MRI reduced the number of strokes that remained cryptogenic at the end of in-hospital diagnostics through the identification of additional potential cardioembolic sources, mainly regional wall motion abnormalities in more than three segments. Furthermore, previously undetected non-acute myocardial infarction was detected in 15% of all cryptogenic stroke patients [45]. Application of cardiac stress MRI is not established in the acute phase of stroke.
Key points
-
Cardiac MRI is a complementary imaging method for work-up of specific cardiac pathologies, for example tumors, cardiomyopathies and left ventricular thrombi or prior myocardial infarction.**
-
Cardiac MRI is not optimal for the evaluation of PFO or the detection of endocarditis.
-
In general, cardiac MRI requires comparatively long image acquisition times and affords the patient to follow commands, limiting the feasibility in acute stroke patients.*
Left atrial imaging in stroke patients
The importance of left atrial imaging increases as the concept and clinical relevance of atrial (cardio-) myopathy evolves. Atrial cardiomyopathy is defined by the presence of structural, architectural, contractile or electrophysiological changes and may carry an increased risk of embolic stroke independent of (ECG detected) AF [73, 74]. Anatomical and functional parameters of the left atrium add prognostic information beyond established risk markers of increased mortality in the older community [75]. Left atrial dilatation is associated with increased stroke risk [76]. In addition, LAA size and morphology are also associated with increased stroke risk [77]. Thus, the characterization of LAA morphology by 3D imaging techniques to detect potential thrombi in the LAA lobi is important in post stroke patients. One problem in this patient cohort is, that a thrombus formation may not be detectable anymore at the moment of the imaging procedure, as the thrombus has led to stroke or residual thrombus formation dissolved spontaneously or by antithrombotic treatment. The target of TOE imaging in this scenario is the analysis of the functional state of the LAA, mainly characterized by LAA emptying velocities. Left atrial shape is another variable to assess thrombogenicity [78, 79]. The application of contrast agents can help differentiate between spontaneous echo contrast and solid thrombi. Tissue Doppler derived markers and strain imaging are promising methods to assess atrial performance and help quantify atrial cardiomyopathy. New echocardiographic techniques include left atrial reservoir strain, which is easy to perform and adds prognostic information, as reported in patients with AF [80]. Decreased left atrial strain is thought to reflect left atrial fibrosis [81,82,83]. LA function can also be measured using cardiac MRI and CT [84,85,86,87]. Ongoing studies like ATTICUS and ARCADIA focus on the potential role of left atrial pathology for recurrent stroke risk after ESUS and investigate, whether secondary stroke prevention using apixaban is superior/non-inferior to acetylsalicylic acid [88, 89]. As (also clinically unapparent) AF leads to atrial fibrosis, left atrial imaging may be applied to tailor intensity of screening for intermittent AF [90].
Key points
-
Left atrial imaging may be used to assess atrial cardiomyopathy.*
-
Presence of atrial cardiomyopathy can help to tailor the intensity of post-stroke monitoring for AF.**
Practical recommendations to use cardiac imaging after ischemic stroke or TIA
Careful work-up is necessary to identify potential cardiac sources of embolism. Furthermore, stroke work-up provides an opportunity to screen for cardiac comorbidities that may prompt further diagnostic evaluation and affect treatment that improves cardiovascular outcome.
In Germany, there is broad consensus to perform echocardiography in selected patients with acute ischemic stroke, as registry data demonstrated an increased use of echocardiography from 62.2% in 2001 to 74.0% in 2006 [91]. This is in line with registry data from Canada showing that the proportion of stroke patients undergoing echocardiography rose from 52% in 2003/2004 to 70% in 2011/2012 [92]. The German Stroke Society (DSG) certification criteria for German Stroke Units require the use of TOE in at least 15% of all ischemic stroke patients (aiming for 20–30%), considering TOE superior to TTE with regard to diagnostic power [93]. The German Cardiac Society (DGK) as well as international societies recommend cardiac MRI or CT in addition to TTE/TOE rather than method of first choice if there is a suspicion for a specific condition/pathology amenable for MRI or CT imaging [19, 60, 94, 95].
An optimal secondary stroke prevention strategy may also affect health economics. However, no prospective randomized study has proven that secondary prevention measurements (e.g. oral anticoagulation) are efficacious with regard to clinically relevant endpoints. Furthermore, there is only limited data available on how often echocardiography leads to relevant changes in therapeutic management. Observational studies, mainly focusing on changes in secondary stroke prevention, suggest that this is only rarely the case, rendering a reduction of stroke recurrence or reduction of other (vascular) endpoints unlikely [37, 38, 96, 97]. However, given the recently published open-label randomized trials demonstrating a benefit of interventional PFO-closure over antiplatelet agents in cryptogenic stroke patients aged 16–60 years [98,99,100], the impact of echocardiography is likely to increase.
Setting for echocardiography
An interdisciplinary team routinely collaborating on the cardiology work-up of post-stroke patients may facilitate cross-discipline communication and improve patient care. Ideally, a specialized cardiovascular imaging suite is nearby or even integrated in the stroke unit, which permits short transportation times or bedside echocardiography. If post stroke echocardiography is performed in a cardiology routine environment, fixed slots for stroke unit patients may facilitate logistics. Echocardiographic work-up should ideally be finalized during the hospital stay to ensure an interdisciplinary approach for patient discussion and potential further diagnostic or therapeutic decisions. Therefore, adequate structures and resources are necessary for stroke-care hospitals. In case of limited echocardiographic capacities and short duration of in-hospital stays, echocardiographic work-up can be performed post-discharge in patients in stable cardiac conditions with an established non-cardiac cause of stroke.
The quality of echocardiographic findings is investigator-dependent [101, 102]. This variability is composed of differences in image acquisition and image analysis [101]. In particular, there seems to be a significant intra- and interobserver variability in the diagnosis and quantification of PFO, spontaneous echo contrast and left atrial thrombi with TOE [103,104,105]. Therefore, TTE and TOE should be performed by an experienced cardiologist, ideally supported by a nurse. A senior cardiology consultant should be available to advise the stroke unit team on specific work-up based on imaging findings.
Key points
-
A standardized set-up and experienced investigators are needed for echocardiographic examinations in stroke patients, which should follow a systematic, standardized protocol.*
-
An interdisciplinary team and standard operating procedures for post-stroke cardiac imaging may enhance decision making and advise further work-up including special cases where cardiac CT or MRI are required.*
Patient selection
Cardiac imaging should be performed if it has potential therapeutic consequences. In case of a defined non-cardiac source of stroke (e.g. arterial dissection) and a low cardiovascular risk profile, cardiac imaging is not essential after stroke or TIA.
Since guidelines recommendation consistently encourage echocardiography in patients with suspected embolic stroke and without contraindications for oral anticoagulation, this approach selects in particular younger patients with stroke or TIA. As cardiac comorbidities such as coronary heart disease, left ventricular hypertrophy, and valvular dysfunction naturally becomes more common in older age groups, expert consensus is that TTE should be considered in patients with at least one established cardiovascular risk factor (Fig. 4).
Expert-based recommendations for post-stroke imaging. Patients with (acute) ischemic stroke or TIA should receive transthoracic echocardiography (TTE) if either a cardiac cause of stroke is suspected or any cardiovascular risk factor is present. Transoesophageal echocardiography (TOE) should additionally be performed in patients with suspicious findings, insufficient image quality or if no cardiac source of stroke was detected despite clinical suspicion for cardioembolic stroke (†TTE and TOE in a single session is recommended if the cardiac source of stroke is suspected as indicated by the bold arrow). Inconclusive TTE or TOE and specific suspicion of cardiac disease may lead to further diagnostic work-up, e.g., contrast-enhanced echocardiography or cardiac magnetic resonance imaging. The value of different cardiovascular imaging methods for common cardiac pathologies is listed in Table 1
Acute ischemic stroke or TIA is an indicator of a comparably high risk of cardiovascular co-morbidities that may not be directly causally related to the present stroke but may significantly affect stroke recurrence rate and survival [106]. In particular, in older patients with acute ischemic stroke, a high cardiovascular risk factor burden is evident [107, 108]. In addition, coronary heart disease is highly prevalent in patients with stroke/TIA or carotid atherosclerosis and should actively be risk-stratified [109,110,111,112]. Cardiac imaging should therefore not be restricted to (comparably young) patients with so far cryptogenic stroke or ESUS [113]. Whereas echocardiographic work-up is generally recommended in TIA patients [114], it is expert consensus that in low-risk TIA patients (i.e. without a history of cardiac disease or stroke, normal cardiac physical examination and normal ECG during monitoring), imaging may seldom result in relevant findings [115]. If a recent cardiologic work-up is documented and clinical status is unaltered, cardiac imaging can be postponed post-hospital discharge, if cardio-embolic source is known already or not expected. Patients at high risk for intracardiac thrombi are individuals with AF which is observed in more than 20% of acute ischemic stroke patients from hospital registries with current rhythm monitoring strategies [85]. However, additional relevant sources of embolism, like a ventricular thrombus or severe atherosclerotic plaques in the aortic arc can be found in AF patients [11, 12]. Furthermore, AF often is associated with additional cardiac abnormalities such as heart failure or valvular heart disease, which are not detected on physical examination, but merit diagnostic attention and therapeutic optimization [11, 17, 18]. TTE should be performed in patients if the presence of heart failure or changes of cardiac function in patients with heart failure would alter further management. The presence of a ventricular thrombus indicates a separate cardiac source of embolism and an LA(A) thrombus may lead to early continuation or starting of oral anticoagulation [11, 12].
Key points
-
Stroke or TIA patients should undergo cardiac imaging, if stroke etiology is uncertain.*
-
Stroke or TIA patients should undergo cardiac imaging, if the presence of pathological findings would alter (medical) management.*
-
Cardiac imaging should be considered in ischemic stroke patients with at least one established cardiovascular risk factor to identify cardiac comorbidities, unless cardiologic work-up within the last 6 months is documented in stroke patients without signs of cardiac dysfunction.
-
Stroke or TIA patients with AF should undergo cardiac imaging for further cardiac work-up, if the presence of a left-sided atrial or ventricular thrombus would alter (medical) management or if a first episode of AF was documented in-hospital.*
Imaging method of choice
Whereas routine echocardiography in all stroke patients is not recommended, guidelines consistently encourage echocardiography in patients with suspected embolic stroke with unsuspicious neurovascular imaging and no contraindications against anticoagulation where a diagnosis would lead to a treatment change [1, 6, 8, 9].
For the diagnosis of atrial sources of emboli TOE is necessary. However, in the vast majority of cases, patients with cardiac thrombi have underlying functional or structural cardiac alterations (e.g. reduced left ventricular ejection fraction for ischemic heart disease or cardiomyopathy, mitral valve stenosis or previously documented AF). If any of these conditions is absent, the 12-lead ECG is normal, and no history of cardiovascular disease is present left atrial thrombi are a rare finding [30, 31, 116]. The diagnosis of PFO has gained more importance, as three recent RCTs demonstrated a benefit of PFO closure over antiplatelet therapy in stroke patients aged 16–60 years with at least moderate shunt [98,99,100]. TOE with an intravenously injection of an agitated air-saline or air-modified gelatin solution (“bubble-test”) is the gold standard of PFO detection and semi-quantification [117]. The extent of intermittent right-left-shunting through the PFO sometimes is difficult to quantify by left atrial bubble transfer, because the opening of the PFO is dependent on the pressure conditions between the right and left atrium. Thus, in the presence of a severe increased left atrial pressure (e.g. severe diastolic dysfunction or severe aortic stenosis) a PFO may not be detectable by bubble passage, even if it is large. Initial screening for a right-to-left shunt can be achieved using contrast transcranial Doppler examination to monitor potential microbubbles in the middle cerebral arteries [118].
Increased left atrial dimensions or parameters of reduced left atrial function summarized as atrial cardiomyopathy may hint towards paroxysmal AF as a potential cause of stroke, reduced left ventricular ejection fraction, and regional wall motion abnormalities towards left ventricular thrombus [14, 73, 119]. Stroke patients with the first episode of AF in-hospital should undergo TTE, as preexisting heart disease underlies newly detected AF according to a cohort study [79, 120].
In stroke patients with known AF prior to stroke, a TTE should be considered (unless cardiac work-up is documented within the last 6 months and cardiac clinical state has not changed). Furthermore, a TOE should be performed in stroke patients with AF, if the presence of an atrial thrombus would alter medical management, e.g. lead to immediate oral anticoagulation despite the risk of secondary hemorrhagic transformation.
Cardiac CT imaging is suitable for the diagnostic classification of patients with suspected coronary artery disease and cardiovascular risk stratification [58,59,60]. However, there is no broad evidence (or consensus), which stroke patients (without suspected endocarditis) may benefit from additional imaging using cardiac CT. Regarding the additional radiation dose, cardiac CT should only be considered as an imaging modality for individual cases in which relevant information cannot be obtained by echocardiography or cardiac MRI [121]. Despite high sensitivity for thrombus detection, long image acquisition times, limited availability (even in centers with specific expertise), needed ability of the patient to follow breathing commands and comparably high costs of cardiac MRI limit its applicability outside the setting of prospective studies. Furthermore, impaired renal function frequently observed in post-stroke patients limits the application of gadolinium-contrast agents or CT-contrast agents [122].
For now, it appears that cardiac CT and MRI may serve as complementary imaging modalities in clinical studies or for in-depth work-up in case of suspected cardiac abnormalities that remain inconclusive in echocardiographic examinations. CT/MRI can also be applied in rare cases where contraindications against TOE are present or image interpretability is impaired. The use of either method will not only depend on the local expertise but largely on the availability of high-quality imaging facilities. One may argue that chest x-ray is routinely accessible in the stroke unit setting, and can reveal cardiac enlargement, pulmonary congestion, pleural effusion and even valve calcifications. As no respective studies are available [123], it is expert consensus that chest x-ray is not sufficient to substitute for echocardiography, cardiac MRI or CT imaging.
Key points
-
Echocardiography is the gold standard imaging modality after ischemic stroke or TIA, if a pathological finding would alter (medical) management.*
-
TOE and TTE should be performed (preferably in a single session) if a cardio-embolic source is deemed a probable cause of ischemic stroke or TIA.
-
TTE should be performed in patients with suspicious findings during TOE, if TOE alone is not sufficient for the comprehensive evaluation of the specific pathology (e.g. regional wall motion abnormalities or apical left ventricular thrombus).
-
TOE should be performed in patients with suspicious findings during TTE, if TTE alone is not sufficient for the comprehensive evaluation of the specific pathology (e.g. endocarditis or assessment of (inter)atrial structures).**
-
TOE should be performed (in patients with so far cryptogenic stroke aged 16–60 years), if PFO presence would alter further management.*
-
TTE should be performed in stroke patients with a first episode of AF in-hospital.*
-
TTE should be considered in ischemic stroke patients with at least one established cardiovascular risk factor unless recent cardiologic work-up is documented.*
-
Cardiac CT should be considered, if there is a suspicion of cardiac or extra cardiac abnormalities, based on other imaging modalities, which are assumed to be clinically relevant. As cardiac CT is associated with radiation exposure, use of cardiac CT should be based on an individual decision.*
-
Cardiac MRI should be considered, if there is a suspicion of left ventricular thrombus or cardiac tumor or in case of an unclear cardiomyopathy after contrast enhanced echocardiography.
Incidental imaging findings
With intensified cardiac imaging, work-up of incidental findings needs to be considered. Such findings may result in uncertainty on diagnostic and treatment consequences in often older and multi-morbid patients. In stroke patients a PFO is a frequent (most often incidental) finding due to the high prevalence in the general population [24]. Frequent incidental findings also include aortic atheroma/plaques, calcification of the aortic/mitral valve or spontaneous echo contrast. Therapeutic decisions should be evidence-based and ideally follow standard operation procedures. An interdisciplinary board may help in efficacious and swift interdisciplinary decisions.
Key points
-
Diagnostic algorithms and interdisciplinary standard operating procedures should be established to avoid over-diagnosis and “over-treatment” because of incidental findings of cardiac imaging.*
Cost-effectiveness of cardiac imaging after stroke
Overall, cost-effectiveness analyses are impaired by the limited data on the effectiveness of treatment for most imaging findings. At present, reliable numbers exist for left atrial appendage thrombus and subsequent OAC only and will probably soon be available for PFO closure [124]. Whereas cost-effectiveness may not be given if performed in unselected acute ischemic stroke patients, modern TTE has been classified as cost-effective compared to no testing when used for suitable indications in post-stroke patients across age groups between 45 and 65 years [1, 4]. These cost calculations neglected a potential benefit from incidental findings related to other common cardiovascular diseases that may be treatable and thus add quality-adjusted life years. No cost–benefit calculations have been performed to assess the overall yield of echocardiographic imaging and treatment changes beyond the initiation of anticoagulation.
For other imaging methods such as cardiac CT or MRI implementation in acute stroke imaging protocols has been suggested, but robust data on the benefit beyond echocardiography are not available. Cardiac CT and in particular MRI examinations are significantly more expensive than echocardiography, with TTE being the cheapest of all cardiac imaging methods [125]. For all imaging modalities, most studies were comparatively small, single-center observational examinations. No systematic head-to-head comparisons including treatment change and outcomes are available. To date, cardiac CT or MRI have not been endorsed as the appropriate utilization of cardiovascular imaging in the setting of stroke [94, 121].
Key points
-
Cost-effectiveness of systematic cardiac imaging in stroke patients should be addressed in prospective trials.
Imaging of the aortic arch
Aortic arch atheromas have been related to recurrent embolic stroke [126]. A CT scan can visualize the whole aortic arch and the descending aorta whereas TOE often is impaired by suboptimal acoustic properties of the aortic arch [64]. Furthermore, novel CT imaging tools permit optimized aortic plaque characterization with delineation of its different components, ulcerations, adherent thrombi beyond plaque thickness and extension and therefore, render CT angiography superior to TOE for aortic plaque assessment [127, 128]. Multi-detector row CT detected atheroma may constitute a risk factor for stroke recurrence, although treatment strategies are not clear yet [127, 129]. MRI angiography can depict aortic atheroma features with limitations for calcifications, mobile structures and ulcerations [130, 131].
Key points
-
A CT angiography scan can visualize the whole aortic arch and the descending aorta whereas TOE often is impaired by suboptimal acoustic properties of the aortic arch.
-
MRI angiography can depict aortic pathology but acquisition is time-consuming, more expensive, and limited with regard to calcifications, mobile structures and ulcerations.*
Summary
In conclusion, stringent high-quality cardiac imaging in ischemic stroke or TIA patients offers the opportunity to reveal stroke causes with direct therapeutic consequences and to improve global cardiovascular risk assessment in a high-risk population for cardiovascular disease. According to the working group, TTE should be considered in stroke patients with at least one established cardiovascular risk factor. A TOE should be performed, if a cardiac source of embolism is suspected or TTE findings need further work-up. Non-invasive cardiac imaging using CT or MRI can be viewed as complementary methods, but should currently be restricted to specific diagnostic questions and clinical studies.
Abbreviations
- ASA:
-
Atrial septal aneurysm
- CT:
-
Computed tomography
- ECG:
-
Electrocardiogram
- ESO:
-
European Stroke Organization
- ESUS:
-
Embolic stroke of undetermined source
- LA:
-
Left atrium
- LAA:
-
Left atrial appendage
- LGE:
-
Late gadolinium enhancement
- MRI:
-
Magnetic resonance imaging
- PFO:
-
Patent foramen ovale
- RCT:
-
Randomized controlled trial
- TIA:
-
Transient ischemic attack
- TOE:
-
Transoesophageal echocardiography
- TTE:
-
Transthoracic echocardiography
References
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2019) Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 50(12):e344–e418. https://doi.org/10.1161/str.0000000000000211
Haeusler KG, Groschel K, Kohrmann M, Anker SD, Brachmann J, Bohm M, Diener HC, Doehner W, Endres M, Gerloff C, Huttner HB, Kaps M, Kirchhof P, Nabavi DG, Nolte CH, Pfeilschifter W, Pieske B, Poli S, Schabitz WR, Thomalla G, Veltkamp R, Steiner T, Laufs U, Rother J, Wachter R, Schnabel R (2018) Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin Res Cardiol 107(10):871–880. https://doi.org/10.1007/s00392-018-1256-9
Camen S, Haeusler KG, Schnabel RB (2019) Cardiac imaging after ischemic stroke: echocardiography, CT, or MRI? Herz 44(4):296–303. https://doi.org/10.1007/s00059-019-4803-x
Holmes M, Rathbone J, Littlewood C, Rawdin A, Stevenson M, Stevens J, Archer R, Evans P, Wang J (2014) Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess (Winchester, England) 18(16):1–176. https://doi.org/10.3310/hta18160
European Stroke Organisation C (2008) Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 25(5):457–507. https://doi.org/10.1159/000131083
Ahmed N, Audebert H, Turc G, Cordonnier C, Christensen H, Sacco S, Sandset EC, Ntaios G, Charidimou A, Toni D, Pristipino C, Kohrmann M, Kuramatsu JB, Thomalla G, Mikulik R, Ford GA, Marti-Fabregas J, Fischer U, Thoren M, Lundstrom E, Rinkel GJ, van der Worp HB, Matusevicius M, Tsivgoulis G, Milionis H, Rubiera M, Hart R, Moreira T, Lantz M, Sjostrand C, Andersen G, Schellinger P, Kostulas K, Sunnerhagen KS, Keselman B, Korompoki E, Purrucker J, Khatri P, Whiteley W, Berge E, Mazya M, Dippel DW, Mustanoja S, Rasmussen M, Soderqvist AK, Escudero-Martinez I, Steiner T (2019) Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11–13 November 2018. Eur Stroke J 4(4):307–317. https://doi.org/10.1177/2396987319863606
Diener HC, Grau AJ, Baldus S, Ghanem A, Groschel K, Liebetrau C, Massberg S, Mollmann H, Nef H, Sander D, Weimar C, Wohrle J, Mattle H (2018) Cryptogenic stroke and patent foramen ovale : S2e guidelines. Nervenarzt 89(10):1143–1153. https://doi.org/10.1007/s00115-018-0609-y
Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, Landeck BF, Maganti K, Michelena HI, Tolstrup K (2016) Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 29(1):1–42. https://doi.org/10.1016/j.echo.2015.09.011
Boulanger JM, Lindsay MP, Gubitz G, Smith EE, Stotts G, Foley N, Bhogal S, Boyle K, Braun L, Goddard T, Heran M, Kanya-Forster N, Lang E, Lavoie P, McClelland M, O’Kelly C, Pageau P, Pettersen J, Purvis H, Shamy M, Tampieri D, vanAdel B, Verbeek R, Blacquiere D, Casaubon L, Ferguson D, Hegedus Y, Jacquin GJ, Kelly M, Kamal N, Linkewich B, Lum C, Mann B, Milot G, Newcommon N, Poirier P, Simpkin W, Snieder E, Trivedi A, Whelan R, Eustace M, Smitko E, Butcher K (2018) Canadian Stroke Best Practice Recommendations for Acute Stroke Management: prehospital, emergency department, and acute inpatient stroke care, 6th edition update 2018. Int J Stroke 13(9):949–984. https://doi.org/10.1177/1747493018786616
Erdur H, Milles LS, Scheitz JF, Villringer K, Haeusler KG, Endres M, Audebert HJ, Fiebach JB, Nolte CH (2019) Clinical significance of acute and chronic ischaemic lesions in multiple cerebral vascular territories. Eur Radiol 29(3):1338–1347. https://doi.org/10.1007/s00330-018-5684-8
Herm J, Konieczny M, Jungehulsing GJ, Endres M, Villringer A, Malzahn U, Heuschmann PU, Haeusler KG (2013) Should transesophageal echocardiography be performed in acute stroke patients with atrial fibrillation? J Clin Neurosci 20(4):554–559. https://doi.org/10.1016/j.jocn.2012.03.049
Steffel J, Collins R, Antz M, Cornu P, Verhamme P, Potpara TS, Albaladejo P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Vanassche T, Potpara T, Camm AJ, Heidbüchel H, External reviewers; Lip GYH, Deneke T, Dagres N, Boriani G, Chao TF, Choi EK, Hils MT, Santos IS, Lane DA, Atar D, Joung B, Cole OM, Field M (2021) European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 25:euab065. https://doi.org/10.1093/eurheartj/ehy136 Online ahead of print
Haeusler KG, Neugebauer H (2019) Closure of patent foramen ovale after ischemic stroke. Dtsch Med Wochenschr 144(22):1561–1569. https://doi.org/10.1055/a-0832-5767
Pathan F, Sivaraj E, Negishi K, Rafiudeen R, Pathan S, D’Elia N, Galligan J, Neilson S, Fonseca R, Marwick TH (2018) Use of atrial strain to predict atrial fibrillation after cerebral ischemia. JACC Cardiovasc Imaging 11(11):1557–1565. https://doi.org/10.1016/j.jcmg.2017.07.027
Sieweke JT, Biber S, Weissenborn K, Heuschmann PU, Akin M, Zauner F, Gabriel MM, Schuppner R, Berliner D, Bauersachs J, Grosse GM, Bavendiek U (2019) Septal total atrial conduction time for prediction of atrial fibrillation in embolic stroke of unknown source: a pilot study. Clin Res Cardiol. https://doi.org/10.1007/s00392-019-01501-2
Olsen FJ, Christensen LM, Krieger DW, Hojberg S, Host N, Karlsen FM, Svendsen JH, Christensen H, Biering-Sorensen T (2019) Relationship between left atrial strain, diastolic dysfunction and subclinical atrial fibrillation in patients with cryptogenic stroke: the SURPRISE echo substudy. Int J Cardiovasc Imaging. https://doi.org/10.1007/s10554-019-01700-y
Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ (2016) Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol 13(3):131–147. https://doi.org/10.1038/nrcardio.2015.191
Thomas KL, Jackson LR 2nd, Shrader P, Ansell J, Fonarow GC, Gersh B, Kowey PR, Mahaffey KW, Singer DE, Thomas L, Piccini JP, Peterson ED (2017) Prevalence, characteristics, and outcomes of valvular heart disease in patients with atrial fibrillation: insights from the ORBIT-AF (Outcomes Registry for Better Informed Treatment for Atrial Fibrillation). J Am Heart Assoc. https://doi.org/10.1161/jaha.117.006475
Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, Habib G, Ringelstein EB, Sicari R, Zamorano JL, Sitges M, Caso P (2010) Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 11(6):461–476. https://doi.org/10.1093/ejechocard/jeq045
Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU (2001) Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 32(12):2735–2740
Strandberg M, Marttila RJ, Helenius H, Hartiala J (2008) Transoesophageal echocardiography should be considered in patients with ischaemic stroke or transient ischaemic attack. Clin Physiol Funct Imaging 28(3):156–160. https://doi.org/10.1111/j.1475-097X.2007.00785.x
de Bruijn SF, Agema WR, Lammers GJ, van der Wall EE, Wolterbeek R, Holman ER, Bollen EL, Bax JJ (2006) Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 37(10):2531–2534. https://doi.org/10.1161/01.str.0000241064.46659.69
Homma S, Sacco RL (2005) Patent foramen ovale and stroke. Circulation 112(7):1063–1072. https://doi.org/10.1161/circulationaha.104.524371
Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y (1988) Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 318(18):1148–1152. https://doi.org/10.1056/nejm198805053181802
Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, Glasgow GL (1988) Patent foramen ovale in young stroke patients. Lancet 2(8601):11–12. https://doi.org/10.1016/s0140-6736(88)92944-3
Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A (2007) Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 357(22):2262–2268. https://doi.org/10.1056/NEJMoa071422
Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, Chedru F, Guerin F, Bousser MG, de Recondo J (1993) Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke 24(12):1865–1873. https://doi.org/10.1161/01.str.24.12.1865
McGrath ER, Paikin JS, Motlagh B, Salehian O, Kapral MK, O’Donnell MJ (2014) Transesophageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J 168(5):706–712. https://doi.org/10.1016/j.ahj.2014.07.025
Katsanos AH, Giannopoulos S, Frogoudaki A, Vrettou AR, Ikonomidis I, Paraskevaidis I, Zompola C, Vadikolias K, Boviatsis E, Parissis J, Voumvourakis K, Kyritsis AP, Tsivgoulis G (2016) The diagnostic yield of transesophageal echocardiography in patients with cryptogenic cerebral ischaemia: a meta-analysis. Eur J Neurol 23(3):569–579. https://doi.org/10.1111/ene.12897
Omran H, Rang B, Schmidt H, Illien S, Schimpf R, Maccarter D, Kubini R, Von Der Recke G, Tiemann K, Becher H, Luderitz B (2000) Incidence of left atrial thrombi in patients in sinus rhythm and with a recent neurologic deficit. Am Heart J 140(4):658–662. https://doi.org/10.1067/mhj.2000.109213
Agmon Y, Khandheria BK, Gentile F, Seward JB (2002) Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation 105(1):27–31
Musolino R, La Spina P, Granata A, Gallitto G, Leggiadro N, Carerj S, Manganaro A, Tripodi F, Epifanio A, Gangemi S, Di Perri R (2003) Ischaemic stroke in young people: a prospective and long-term follow-up study. Cerebrovasc Dis 15(1–2):121–128. https://doi.org/10.1159/000067139
Roijer A, Lindgren A, Algotsson L, Norrving B, Olsson B, Eskilsson J (1997) Cardiac changes in stroke patients and controls evaluated with transoesophageal echocardiography. Scand Cardiovasc J 31(6):329–337. https://doi.org/10.3109/14017439709075949
Strandberg M, Marttila RJ, Helenius H, Hartiala J (2002) Transoesophageal echocardiography in selecting patients for anticoagulation after ischaemic stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry 73(1):29–33
Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ (2005) An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 58(5):688–697. https://doi.org/10.1002/ana.20617
Celeste F, Muratori M, Mapelli M, Pepi M (2017) The evolving role and use of echocardiography in the evaluation of cardiac source of embolism. J Cardiovasc Echogr 27(2):33–44. https://doi.org/10.4103/jcecho.jcecho_1_17
Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A (2006) Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 37(3):859–864. https://doi.org/10.1161/01.STR.0000202592.87021.b7
Cho HJ, Choi HY, Kim YD, Nam HS, Han SW, Ha JW, Chung NS, Heo JH (2010) Transoesophageal echocardiography in patients with acute stroke with sinus rhythm and no cardiac disease history. J Neurol Neurosurg Psychiatry 81(4):412–415. https://doi.org/10.1136/jnnp.2009.190322
Prabhu M, Raju D, Pauli H (2012) Transesophageal echocardiography: instrumentation and system controls. Ann Card Anaesth 15(2):144–155. https://doi.org/10.4103/0971-9784.95080
Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK (2010) Safety of transesophageal echocardiography. J Am Soc Echocardiogr 23(11):1115–1127. https://doi.org/10.1016/j.echo.2010.08.013 (quiz 1220-1111)
Pearson AC, Labovitz AJ, Tatineni S, Gomez CR (1991) Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 17(1):66–72. https://doi.org/10.1016/0735-1097(91)90705-e
Mayo JR, Leipsic JA (2009) Radiation dose in cardiac CT. AJR Am J Roentgenol 192(3):646–653. https://doi.org/10.2214/ajr.08.2066
Menke J, Unterberg-Buchwald C, Staab W, Sohns JM, Seif Amir Hosseini A, Schwarz A (2013) Head-to-head comparison of prospectively triggered vs retrospectively gated coronary computed tomography angiography: meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am Heart J 165(2):154-163.e153. https://doi.org/10.1016/j.ahj.2012.10.026
Stocker TJ, Deseive S, Leipsic J, Hadamitzky M, Chen MY, Rubinshtein R, Heckner M, Bax JJ, Fang XM, Grove EL, Lesser J, Maurovich-Horvat P, Otton J, Shin S, Pontone G, Marques H, Chow B, Nomura CH, Tabbalat R, Schmermund A, Kang JW, Naoum C, Atkins M, Martuscelli E, Massberg S, Hausleiter J (2018) Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J 39(41):3715–3723. https://doi.org/10.1093/eurheartj/ehy546
Haeusler KG, Wollboldt C, Bentheim LZ, Herm J, Jager S, Kunze C, Eberle HC, Deluigi CC, Bruder O, Malsch C, Heuschmann PU, Endres M, Audebert HJ, Morguet AJ, Jensen C, Fiebach JB (2017) Feasibility and diagnostic value of cardiovascular magnetic resonance imaging after acute ischemic stroke of undetermined origin. Stroke 48(5):1241–1247. https://doi.org/10.1161/STROKEAHA.116.016227
Nikolaou K, Flohr T, Knez A, Rist C, Wintersperger B, Johnson T, Reiser MF, Becker CR (2004) Advances in cardiac CT imaging: 64-slice scanner. Int J Cardiovasc Imaging 20(6):535–540. https://doi.org/10.1007/s10554-004-7015-1
Schmermund A, Marwan M, Hausleiter J, Barth S, Bruder O, Kerber S, Korosoglou G, Leber A, Moshage W, Schröder S, Schneider S, Senges J, Achenbach S (2017) Declining radiation dose of coronary computed tomography angiography: German cardiac CT registry experience 2009–2014. Clin Res Cardiol 106(11):905–912. https://doi.org/10.1007/s00392-017-1136-8
Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ (2013) Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 6(2):185–194. https://doi.org/10.1161/circimaging.112.000153
Kim SC, Chun EJ, Choi SI, Lee SJ, Chang HJ, Han MK, Bae HJ, Park JH (2010) Differentiation between spontaneous echocardiographic contrast and left atrial appendage thrombus in patients with suspected embolic stroke using two-phase multidetector computed tomography. Am J Cardiol 106(8):1174–1181. https://doi.org/10.1016/j.amjcard.2010.06.033
Hur J, Kim YJ, Lee HJ, Nam JE, Hong YJ, Kim HY, Lee JW, Choi BW (2012) Cardioembolic stroke: dual-energy cardiac CT for differentiation of left atrial appendage thrombus and circulatory stasis. Radiology 263(3):688–695. https://doi.org/10.1148/radiol.12111691
Bittencourt MS, Achenbach S, Marwan M, Seltmann M, Muschiol G, Ropers D, Daniel WG, Pflederer T (2012) Left ventricular thrombus attenuation characterization in cardiac computed tomography angiography. J Cardiovasc Comput Tomogr 6(2):121–126. https://doi.org/10.1016/j.jcct.2011.12.006
Malik SB, Chen N, Parker RA 3rd, Hsu JY (2017) Transthoracic echocardiography: pitfalls and limitations as delineated at cardiac CT and MR imaging. Radiographics 37(2):383–406. https://doi.org/10.1148/rg.2017160105
Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, Shim CY, Kim TH, Nam JE, Choe KO, Choi BW (2009) Cardiac computed tomographic angiography for detection of cardiac sources of embolism in stroke patients. Stroke 40(6):2073–2078. https://doi.org/10.1161/strokeaha.108.537928
Habets J, Tanis W, Reitsma JB, van den Brink RB, Mali WP, Chamuleau SA, Budde RP (2015) Are novel non-invasive imaging techniques needed in patients with suspected prosthetic heart valve endocarditis? A systematic review and meta-analysis. Eur Radiol 25(7):2125–2133. https://doi.org/10.1007/s00330-015-3605-7
Bruun NE, Habib G, Thuny F, Sogaard P (2014) Cardiac imaging in infectious endocarditis. Eur Heart J 35(10):624–632. https://doi.org/10.1093/eurheartj/eht274
Higgins J, Mayo J, Skarsgard P (2013) Cardiac computed tomography facilitates operative planning in patients with mitral calcification. Ann Thorac Surg 95(1):e9-11. https://doi.org/10.1016/j.athoracsur.2012.07.059
Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC (2018) Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 379(10):924–933. https://doi.org/10.1056/NEJMoa1805971
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ (2020) 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 41(3):407–477. https://doi.org/10.1093/eurheartj/ehz425
Hur J, Lee KH, Hong SR, Suh YJ, Hong YJ, Lee HJ, Kim YJ, Lee HS, Chang HJ, Choi BW (2015) Prognostic value of coronary computed tomography angiography in stroke patients. Atherosclerosis 238(2):271–277. https://doi.org/10.1016/j.atherosclerosis.2014.10.102
Achenbach S, Barkhausen J, Beer M, Beerbaum P, Dill T, Eichhorn J, Fratz S, Gutberlet M, Hoffmann M, Huber A, Hunold P, Klein C, Krombach G, Kreitner KF, Kuhne T, Lotz J, Maintz D, Mahrholdt H, Merkle N, Messroghli D, Miller S, Paetsch I, Radke P, Steen H, Thiele H, Sarikouch S, Fischbach R (2012) Consensus recommendations of the German Radiology Society (DRG), the German Cardiac Society (DGK) and the German Society for Pediatric Cardiology (DGPK) on the use of cardiac imaging with computed tomography and magnetic resonance imaging. Rofo 184(4):345–368. https://doi.org/10.1055/s-0031-1299400
Sipola P, Hedman M, Onatsu J, Turpeinen A, Halinen M, Jakala P, Vanninen R (2013) Computed tomography and echocardiography together reveal more high-risk findings than echocardiography alone in the diagnostics of stroke etiology. Cerebrovasc Dis 35(6):521–530. https://doi.org/10.1159/000350734
Boussel L, Cakmak S, Wintermark M, Nighoghossian N, Loffroy R, Coulon P, Derex L, Cho TH, Douek PC (2011) Ischemic stroke: etiologic work-up with multidetector CT of heart and extra- and intracranial arteries. Radiology 258(1):206–212. https://doi.org/10.1148/radiol.10100804
Yeo LLL, Holmin S, Andersson T, Lundstrom E, Gopinathan A, Lim EL, Leong BSH, Kuan WS, Ting E, Tan BYQ, Eide SE, Tay ELK (2017) Nongated cardiac computed tomographic angiograms for detection of embolic sources in acute ischemic stroke. Stroke 48(5):1256–1261. https://doi.org/10.1161/strokeaha.117.016903
Chatzikonstantinou A, Krissak R, Fluchter S, Artemis D, Schaefer A, Schoenberg SO, Hennerici MG, Fink C (2012) CT angiography of the aorta is superior to transesophageal echocardiography for determining stroke subtypes in patients with cryptogenic ischemic stroke. Cerebrovasc Dis 33(4):322–328. https://doi.org/10.1159/000335828
Haeusler KG, Jensen C, Scheitz JF, Krause T, Wollboldt C, Witzenbichler B, Audebert HJ, Landmesser U, Fiebach JB, Nolte CH, Endres M, Mochmann HC (2019) Cardiac magnetic resonance imaging in patients with acute ischemic stroke and elevated troponin: a TRoponin ELevation in Acute Ischemic Stroke (TRELAS) Sub-Study. Cerebrovasc Dis Extra 9(1):19–24. https://doi.org/10.1159/000498864
Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, Brosnan R, Shah DJ, Velazquez EJ, Parker M, Judd RM, Kim RJ (2011) LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 4(7):702–712. https://doi.org/10.1016/j.jcmg.2011.03.017
Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M, Velazquez EJ, Steenbergen C, Judd RM, Kim RJ (2008) Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 52(2):148–157. https://doi.org/10.1016/j.jacc.2008.03.041
Weinsaft JW, Kim J, Medicherla CB, Ma CL, Codella NC, Kukar N, Alaref S, Kim RJ, Devereux RB (2016) Echocardiographic algorithm for post-myocardial infarction LV thrombus: a gatekeeper for thrombus evaluation by delayed enhancement CMR. JACC Cardiovasc Imaging 9(5):505–515. https://doi.org/10.1016/j.jcmg.2015.06.017
Captur G, Nihoyannopoulos P (2010) Left ventricular non-compaction: genetic heterogeneity, diagnosis and clinical course. Int J Cardiol 140(2):145–153. https://doi.org/10.1016/j.ijcard.2009.07.003
Fontana M, Chung R, Hawkins PN, Moon JC (2015) Cardiovascular magnetic resonance for amyloidosis. Heart Fail Rev 20(2):133–144. https://doi.org/10.1007/s10741-014-9470-7
Fonseca AC, Marto JP, Pimenta D, Guimaraes T, Alves PN, Inacio N, Viana-Baptista M, Pinho EMT, Pinto FJ, Ferro JM, Almeida AG (2019) Undetermined stroke genesis and hidden cardiomyopathies determined by cardiac magnetic resonance. Neurology. https://doi.org/10.1212/wnl.0000000000008698
Ohyama H, Hosomi N, Takahashi T, Mizushige K, Osaka K, Kohno M, Koziol JA (2003) Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke 34(10):2436–2439. https://doi.org/10.1161/01.str.0000090350.73614.0f
Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S (2016) EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 18(10):1455–1490. https://doi.org/10.1093/europace/euw161
Hoit BD (2014) Left atrial size and function: role in prognosis. J Am Coll Cardiol 63(6):493–505. https://doi.org/10.1016/j.jacc.2013.10.055
Ramkumar S, Ochi A, Kawakami H, Yang H, Potter EL, D’Elia N, Negishi T, Negishi K, Marwick TH (2019) Echocardiographic Risk Assessment to Guide Screening for Atrial Fibrillation. J Am Soc Echocardiogr 32(10):1259–1267. https://doi.org/10.1016/j.echo.2019.07.003
Froehlich L, Meyre P, Aeschbacher S, Blum S, Djokic D, Kuehne M, Osswald S, Kaufmann BA, Conen D (2019) Left atrial dimension and cardiovascular outcomes in patients with and without atrial fibrillation: a systematic review and meta-analysis. Heart 105(24):1884–1891. https://doi.org/10.1136/heartjnl-2019-315174
Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F (2012) Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 60(6):531–538. https://doi.org/10.1016/j.jacc.2012.04.032
Handke M, Harloff A, Hetzel A, Olschewski M, Bode C, Geibel A (2005) Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation–a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J Am Soc Echocardiogr 18(12):1366–1372. https://doi.org/10.1016/j.echo.2005.05.006
Donal E, Lip GY, Galderisi M, Goette A, Shah D, Marwan M, Lederlin M, Mondillo S, Edvardsen T, Sitges M, Grapsa J, Garbi M, Senior R, Gimelli A, Potpara TS, Van Gelder IC, Gorenek B, Mabo P, Lancellotti P, Kuck KH, Popescu BA, Hindricks G, Habib G, Cardim NM, Cosyns B, Delgado V, Haugaa KH, Muraru D, Nieman K, Boriani G, Cohen A (2016) EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging 17(4):355–383. https://doi.org/10.1093/ehjci/jev354
Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF (2011) Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol 57(7):831–838. https://doi.org/10.1016/j.jacc.2010.09.049
Stamboul K, Lorin J, Lorgis L, Guenancia C, Beer JC, Touzery C, Rochette L, Vergely C, Cottin Y, Zeller M (2015) Atrial fibrillation is associated with a marker of endothelial function and oxidative stress in patients with acute myocardial infarction. PLoS ONE 10(7):e0131439. https://doi.org/10.1371/journal.pone.0131439
Leong DP, Joyce E, Debonnaire P, Katsanos S, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA (2017) Left atrial dysfunction in the pathogenesis of cryptogenic stroke: novel insights from speckle-tracking echocardiography. J Am Soc Echocardiogr 30(1):71-79.e71. https://doi.org/10.1016/j.echo.2016.09.013
Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF (2010) Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 3(3):231–239. https://doi.org/10.1161/circimaging.109.865683
Yaghi S, Song C, Gray WA, Furie KL, Elkind MS, Kamel H (2015) Left atrial appendage function and stroke risk. Stroke 46(12):3554–3559. https://doi.org/10.1161/STROKEAHA.115.011273
Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, Brandes A, Bustamante A, Casadei B, Crijns H, Doehner W, Engstrom G, Fauchier L, Friberg L, Gladstone DJ, Glotzer TV, Goto S, Hankey GJ, Harbison JA, Hobbs FDR, Johnson LSB, Kamel H, Kirchhof P, Korompoki E, Krieger DW, Lip GYH, Lochen ML, Mairesse GH, Montaner J, Neubeck L, Ntaios G, Piccini JP, Potpara TS, Quinn TJ, Reiffel JA, Ribeiro ALP, Rienstra M, Rosenqvist M, Sakis T, Sinner MF, Svendsen JH, Van Gelder IC, Wachter R, Wijeratne T, Yan B (2019) Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN International Collaboration. Circulation 140(22):1834–1850. https://doi.org/10.1161/circulationaha.119.040267
Kuhl JT, Lonborg J, Fuchs A, Andersen MJ, Vejlstrup N, Kelbaek H, Engstrom T, Moller JE, Kofoed KF (2012) Assessment of left atrial volume and function: a comparative study between echocardiography, magnetic resonance imaging and multi slice computed tomography. Int J Cardiovasc Imaging 28(5):1061–1071. https://doi.org/10.1007/s10554-011-9930-2
Olsen FJ, Bertelsen L, de Knegt MC, Christensen TE, Vejlstrup N, Svendsen JH, Jensen JS, Biering-Sorensen T (2016) Multimodality cardiac imaging for the assessment of left atrial function and the association with atrial arrhythmias. Circu Cardiovasc imaging. https://doi.org/10.1161/circimaging.116.004947
Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nagele T, Brachmann J, Jung W, Gahn G, Schmid E, Baezner H, Keller T, Petzold GC, Schrickel JW, Liman J, Wachter R, Schon F, Schabet M, Lindner A, Ludolph AC, Kimmig H, Jander S, Schlegel U, Gawaz M, Ziemann U (2017) Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): rationale and study design. Int J Stroke 12(9):985–990. https://doi.org/10.1177/1747493016681019
Kamel H, Longstreth WT Jr, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA, Di Tullio MR, Hod EA, Soliman EZ, Chaturvedi S, Moy CS, Janis S, Elkind MS (2019) The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke 14(2):207–214. https://doi.org/10.1177/1747493018799981
Poli S, Diedler J, Hartig F, Gotz N, Bauer A, Sachse T, Muller K, Muller I, Stimpfle F, Duckheim M, Steeg M, Eick C, Schreieck J, Gawaz M, Ziemann U, Zuern CS (2016) Insertable cardiac monitors after cryptogenic stroke–a risk factor based approach to enhance the detection rate for paroxysmal atrial fibrillation. Eur J Neurol 23(2):375–381. https://doi.org/10.1111/ene.12843
Grau AJ, Eicke M, Biegler MK, Faldum A, Bamberg C, Haass A, Hardt R, Hufschmidt A, Lowitzsch K, Marx J, Schmitt E, Schoenemann H, von Arnim W, Weiss H, Dienlin S (2010) Quality monitoring of acute stroke care in Rhineland-Palatinate, Germany, 2001–2006. Stroke 41(7):1495–1500. https://doi.org/10.1161/strokeaha.110.582239
Ng VT, Bayoumi AM, Fang J, Burton KR, Stamplecoski M, Edwards JD, Kapral MK (2016) Temporal trends in the use of investigations after stroke or transient ischemic attack. Med Care 54(5):430–434. https://doi.org/10.1097/mlr.0000000000000499
Nabavi DG, Koennecke HC, Ossenbrink M, Grau A, Busse O (2019) Certification criteria for stroke units in Germany : Update 2018. Nervenarzt 90(4):335–342. https://doi.org/10.1007/s00115-018-0633-y
Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, Kramer CM, Lesser JR, Martin ET, Messer JV, Redberg RF, Rubin GD, Rumsfeld JS, Taylor AJ, Weigold WG, Woodard PK, Brindis RG, Hendel RC, Douglas PS, Peterson ED, Wolk MJ, Allen JM, Patel MR (2006) ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 48(7):1475–1497. https://doi.org/10.1016/j.jacc.2006.07.003
Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P, Dehmer GJ, Doherty JU, Schoenhagen P, Bashore TM, Bhave NM, Calnon DA, Carabello B, Conte J, Dickfeld T, Edmundowicz D, Ferrari VA, Hall ME, Ghoshhajra B, Mehrotra P, Naqvi TZ, Reece TB, Starling RC, Szerlip M, Tzou WS, Wong JB (2019) ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 Appropriate Use Criteria for Multimodality Imaging in the Assessment of Cardiac Structure and Function in Nonvalvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Am Coll Cardiol 73(4):488–516. https://doi.org/10.1016/j.jacc.2018.10.038
Wasser K, Weber-Kruger M, Jurries F, Liman J, Hamann GF, Kermer P, Uphaus T, Protsenko E, Seegers J, Mende M, Groschel K, Wachter R (2019) The cardiac diagnostic work-up in stroke patients—a subanalysis of the Find-AFRANDOMISED trial. PLoS ONE 14(5):e0216530. https://doi.org/10.1371/journal.pone.0216530
Douen A, Pageau N, Medic S (2007) Usefulness of cardiovascular investigations in stroke management: clinical relevance and economic implications. Stroke 38(6):1956–1958. https://doi.org/10.1161/strokeaha.106.477760
Sondergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjostrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L (2017) Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 377(11):1033–1042. https://doi.org/10.1056/NEJMoa1707404
Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Bejot Y, Vuillier F, Detante O, Guidoux C, Canaple S, Vaduva C, Dequatre-Ponchelle N, Sibon I, Garnier P, Ferrier A, Timsit S, Robinet-Borgomano E, Sablot D, Lacour JC, Zuber M, Favrole P, Pinel JF, Apoil M, Reiner P, Lefebvre C, Guerin P, Piot C, Rossi R, Dubois-Rande JL, Eicher JC, Meneveau N, Lusson JR, Bertrand B, Schleich JM, Godart F, Thambo JB, Leborgne L, Michel P, Pierard L, Turc G, Barthelet M, Charles-Nelson A, Weimar C, Moulin T, Juliard JM, Chatellier G (2017) Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 377(11):1011–1021. https://doi.org/10.1056/NEJMoa1705915
Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, Song JM, Kang DH, Kwon SU, Kang DW, Lee D, Kwon HS, Yun SC, Sun BJ, Park JH, Lee JH, Jeong HS, Song HJ, Kim J, Park SJ (2018) Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO Trial. J Am Coll Cardiol 71(20):2335–2342. https://doi.org/10.1016/j.jacc.2018.02.046
Thorstensen A, Dalen H, Amundsen BH, Aase SA, Stoylen A (2010) Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr 11(2):149–156. https://doi.org/10.1093/ejechocard/jep188
Otterstad JE, Froeland G, St John Sutton M, Holme I (1997) Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J 18(3):507–513. https://doi.org/10.1093/oxfordjournals.eurheartj.a015273
Cabanes L, Coste J, Derumeaux G, Jeanrenaud X, Lamy C, Zuber M, Mas JL (2002) Interobserver and intraobserver variability in detection of patent foramen ovale and atrial septal aneurysm with transesophageal echocardiography. J Am Soc Echocardiogr 15(5):441–446. https://doi.org/10.1067/mje.2002.116718
Kronik G, Stollberger C, Schuh M, Abzieher F, Slany J, Schneider B (1995) Interobserver variability in the detection of spontaneous echo contrast, left atrial thrombi, and left atrial appendage thrombi by transoesophageal echocardiography. Br Heart J 74(1):80–83. https://doi.org/10.1136/hrt.74.1.80
Schneider B, Stollberger C, Schneider B (2007) Diagnosis of left atrial appendage thrombi by multiplane transesophageal echocardiography: interlaboratory comparative study. Circ J 71(1):122–125. https://doi.org/10.1253/circj.71.122
Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, Stewart-Wynne EG (2000) Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke 31(9):2080–2086. https://doi.org/10.1161/01.str.31.9.2080
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS (2019) Heart disease and stroke statistics-2019 update: a report From the American Heart Association. Circulation 139(10):e56–e528. https://doi.org/10.1161/cir.0000000000000659
Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC (2001) Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 32(11):2559–2566. https://doi.org/10.1161/hs1101.098524
Polak JF, Szklo M, O’Leary DH (2017) Carotid intima-media thickness score, positive coronary artery calcium score, and incident coronary heart disease: the multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. https://doi.org/10.1161/jaha.116.004612
Selwaness M, Bos D, van den Bouwhuijsen Q, Portegies ML, Ikram MA, Hofman A, Franco OH, van der Lugt A, Wentzel JJ, Vernooij MW (2016) Carotid atherosclerotic plaque characteristics on magnetic resonance imaging relate with history of stroke and coronary heart disease. Stroke 47(6):1542–1547. https://doi.org/10.1161/strokeaha.116.012923
Calvet D, Touze E, Varenne O, Sablayrolles JL, Weber S, Mas JL (2010) Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation 121(14):1623–1629. https://doi.org/10.1161/circulationaha.109.906958
Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, Taubert KA (2003) Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation 108(10):1278–1290. https://doi.org/10.1161/01.cir.0000090444.87006.cf
Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ (2017) Embolic stroke of undetermined source: a systematic review and clinical update. Stroke 48(4):867–872. https://doi.org/10.1161/strokeaha.116.016414
Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL (2009) Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 40(6):2276–2293. https://doi.org/10.1161/strokeaha.108.192218
Harmouche E, Mahmoud GA, Ross M, Hockenberry J, Dharia R, Nahab F (2017) Early echocardiography has a low yield in patients with transient ischemic attack. J Stroke Cerebrovasc Dis 26(8):1858–1863. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.04.022
Leung DY, Black IW, Cranney GB, Walsh WF, Grimm RA, Stewart WJ, Thomas JD (1995) Selection of patients for transesophageal echocardiography after stroke and systemic embolic events. Role of transthoracic echocardiography. Stroke 26(10):1820–1824
Maffe S, Dellavesa P, Zenone F, Paino AM, Paffoni P, Perucca A, Kozel D, Signorotti F, Bielli M, Parravicini U, Pardo NF, Cucchi L, Aymele AG, Zanetta M (2010) Transthoracic second harmonic two- and three-dimensional echocardiography for detection of patent foramen ovale. Eur J Echocardiogr 11(1):57–63. https://doi.org/10.1093/ejechocard/jep165
Mojadidi MK, Roberts SC, Winoker JS, Romero J, Goodman-Meza D, Gevorgyan R, Tobis JM (2014) Accuracy of transcranial Doppler for the diagnosis of intracardiac right-to-left shunt: a bivariate meta-analysis of prospective studies. JACC Cardiovasc Imaging 7(3):236–250. https://doi.org/10.1016/j.jcmg.2013.12.011
Takasugi J, Yamagami H, Noguchi T, Morita Y, Tanaka T, Okuno Y, Yasuda S, Toyoda K, Gon Y, Todo K, Sakaguchi M, Nagatsuka K (2017) Detection of left ventricular thrombus by cardiac magnetic resonance in embolic stroke of undetermined source. Stroke 48(9):2434–2440. https://doi.org/10.1161/strokeaha.117.018263
Rizos T, Horstmann S, Dittgen F, Tager T, Jenetzky E, Heuschmann P, Veltkamp R (2016) Preexisting heart disease underlies newly diagnosed atrial fibrillation after acute ischemic stroke. Stroke 47(2):336–341. https://doi.org/10.1161/STROKEAHA.115.011465
Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC Jr (2010) ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 56(22):1864–1894. https://doi.org/10.1016/j.jacc.2010.07.005
Pereg D, Rozenbaum Z, Vorobeichik D, Shlomo N, Gilad R, Bloch S, Mosseri M, Tanne D (2016) Prevalence and significance of unrecognized renal dysfunction in patients with stroke. Am J Med 129(10):1074–1081. https://doi.org/10.1016/j.amjmed.2016.05.003
Fonseca C, Mota T, Morais H, Matias F, Costa C, Oliveira AG, Ceia F (2004) The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail 6(6):807–812. https://doi.org/10.1016/j.ejheart.2004.09.004 (821-802)
Leppert MH, Poisson SN, Carroll JD, Thaler DE, Kim CH, Orjuela KD, Ho PM, Burke JF, Campbell JD (2018) Cost-effectiveness of patent foramen ovale closure versus medical therapy for secondary stroke prevention. Stroke 49(6):1443–1450. https://doi.org/10.1161/strokeaha.117.020322
Pagan RJ, Parikh PP, Mergo PJ, Gerber TC, Mankad R, Freeman WD, Shapiro BP (2015) Emerging role of cardiovascular CT and MRI in the evaluation of stroke. AJR Am J Roentgenol 204(2):269–280. https://doi.org/10.2214/ajr.14.13051
Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S (2009) Aortic arch plaques and risk of recurrent stroke and death. Circulation 119(17):2376–2382. https://doi.org/10.1161/circulationaha.108.811935
Ko Y, Kim WJ, Jang MS, Yang MH, Park JH, Choi SI, Chun EJ, Lee SJ, Han MK, Bae HJ (2012) Is aortic atherothrombotic disease detected using multidetector-row CT associated with an increased risk of early ischemic lesion recurrence after acute ischemic stroke? Stroke 43(3):764–769. https://doi.org/10.1161/strokeaha.111.632182
Hur J, Kim YJ, Lee HJ, Nam JE, Ha JW, Heo JH, Chang HJ, Kim HS, Hong YJ, Kim HY, Choe KO, Choi BW (2011) Dual-enhanced cardiac CT for detection of left atrial appendage thrombus in patients with stroke: a prospective comparison study with transesophageal echocardiography. Stroke 42(9):2471–2477. https://doi.org/10.1161/strokeaha.110.611293
Zavala JA, Amarrenco P, Davis SM, Jones EF, Young D, Macleod MR, Horky LL, Donnan GA (2006) Aortic arch atheroma. Int J Stroke 1(2):74–80. https://doi.org/10.1111/j.1747-4949.2006.00026.x
Fayad ZA, Nahar T, Fallon JT, Goldman M, Aguinaldo JG, Badimon JJ, Shinnar M, Chesebro JH, Fuster V (2000) In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation 101(21):2503–2509. https://doi.org/10.1161/01.cir.101.21.2503
Faber T, Rippy A, Hyslop WB, Hinderliter A, Sen S (2013) Cardiovascular MRI in detection and measurement of aortic atheroma in Stroke/TIA patients. J Neurol Disord 1(4):139. https://doi.org/10.4172/2329-6895.1000139
Acknowledgements
This expert opinion paper on Cardiac imaging after acute ischemic stroke or transient ischemic attack (TIA) includes a statement of the “Heart and Brain” consortium of the German Cardiac Society and the German Stroke Society. The standardized echocardiographic work-up as outlined has been set up in context with studies of the German Centre for Cardiovascular Research (Deutsches Zentrum für Herz-Kreislauf-Forschung, DZHK, e.V.).
Funding
Open Access funding enabled and organized by projekt DEAL. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RBS has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 648131, from the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 847770 (AFFECT-EU) and German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239). RBS has received speaker honoraria and consulting fees from BMS/Pfizer outside this work. FK reports lecture/advisory board fees from Novartis, Bracco, AstraZeneca, Bayer, Alnylam, Sanofi, Shire, Pfizer, Akcea, Canon, all outside this manuscript. UB reports lecture fees/advisory board fees from Abott, Alnylam, Amgen, Astra Zeneca, Novartis outside the submitted work and travel support from Amgen, Bayer, Berlin-Chemie and Pfizer. MB reports fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Servier, Medtronic, ReCor, Vifor, Novartis and Abbott. MB is supported by the Deutsche Forschungsgemeinschaft (DFG, TTR 219, S-01). ME reports grants from Bayer and fees paid to the Charité from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis, Pfizer, all outside the submitted work. HBH reports research grants by Novartis, Medtronic, UCB Pharma and Portola Pharmaceuticals. HBH reports personal fees from Bayer AG, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, CLS Behring, UCB Pharma and Portola Pharmaceuticals. JL reports lecture fees/advisory board fees from Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb, Daiichi Sankyo, Stryker and Pfizer. CM reports research cooperation with the University of Würzburg and Tomtec Imaging Systems funded by a research grant from the Bavarian Ministry of Economic Affairs, Regional Development and Energy, Germany; advisory and speakers honoraria as well as travel grants from Amgen, Tomtec, Orion Pharma, Alnylam, AKCEA, Pfizer, and EBR Systems; principal investigator in trials sponsored by Alnylam and AstraZeneca; financial support from the interdisciplinary center for clinical research—IZKF Würzburg (advanced clinician-scientist program).WP received honoraria and lecture fees from Bayer Healthcare, Pfizer, Stryker neurovascular and research grants from Boehringer Ingelheim and Stryker neurovascular. SP received speaker’s/consulting honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb/Pizer, Daiichi Sankyo, and Werfen, reimbursement for congress traveling from Bayer, and Boehringer-Ingelheim, and research support from Bristol-Myers Squibb/Pizer, Boehringer-Ingelheim, Daiichi Sankyo, and Helena Laboratories (all outside of the present work). TR received consulting honoraria, speakers’ honoraria and travel support from Bristol-Myers Squibb/Pfizer, Boehringer-Ingelheim, Bayer HealthCare and DaichiiSankyo, outside the submitted work. AR reports lecture honoraria from Pfizer, Boehringer Ingelheim, Bayer, BMS, Berlin Chemie. JR reports lecture fees/advisory board fees from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, Pfizer (unrelated to the submitted manuscript). WRS reports research grant Health Economic Research Zentrum, Ferrer. Speakers’ Bureau: Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo Co., Bayer, Pfizer, Medtronic, Ferrer as well as consultant/advisory board fees from Boehringer Ingelheim, Daiichi Sankyo Co., Medtronic. GT reports study grants by Bayer, lecture fees/advisory board fees from Acandis, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb / Pfizer, Daiichi Sankyo, and Stryker. RW reports grants from Bundesministerium für Bildung und Forschung (BMBF), Deutsches Zentrum für Herz-/Kreislaufforschung, Deutsche Forschungsgemeinschaft, European Union and Medtronic, all outside the submitted work. He received personal fees from AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers-Squibb, CVRX, Daiichi Sankyo, Gilead, Medtronic Novartis, Pfizer, Pharmacosmos, Servier, outside the submitted work. KGH reports study grants by Bayer and Sanofi-Aventis, lecture fees/advisory board fees from Abbott, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Bristol-Myers-Squibb, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Pfizer, Premier Research and Sanofi-Aventis. The other authors report no conflict of interest. Sollte formal vor den COIs von KGH aufgeführt werden.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schnabel, R.B., Camen, S., Knebel, F. et al. Expert opinion paper on cardiac imaging after ischemic stroke. Clin Res Cardiol 110, 938–958 (2021). https://doi.org/10.1007/s00392-021-01834-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01834-x