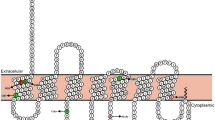
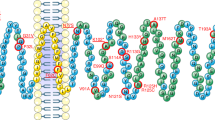
Abstract
Mutations in the melanocortin 4 receptor gene (MC4R) are associated with obesity but little is known about the prevalence and impact of such mutations throughout human growth and development. We examined the MC4R coding sequence in 5,724 participants from the Avon Longitudinal Study of Parents and Children, functionally characterized all nonsynonymous MC4R variants and examined their association with anthropometric phenotypes from childhood to early adulthood. The frequency of heterozygous loss-of-function (LoF) mutations in MC4R was ~1 in 337 (0.30%), considerably higher than previous estimates. At age 18 years, mean differences in body weight, body mass index and fat mass between carriers and noncarriers of LoF mutations were 17.76 kg (95% CI 9.41, 26.10), 4.84 kg m−2 (95% CI 2.19, 7.49) and 14.78 kg (95% CI 8.56, 20.99), respectively. MC4R LoF mutations may be more common than previously reported and carriers of such variants may enter adult life with a substantial burden of excess adiposity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Full details of the cohort and study design have been described previously and are available at http://www.alspac.bris.ac.uk. Please note that the study website contains details of all the data that are available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/). ALSPAC data are available through a system of managed open access. Data for this project were accessed under the project number B2891. The application steps for ALSPAC data access are below.
(1) Please read the ALSPAC access policy, which describes the process of accessing the data in detail and outlines the costs associated with doing so.
(2) You may also find it useful to browse the fully searchable research proposals database, which lists all research projects that have been approved since April 2011.
(3) Please submit your research proposal for consideration by the ALSPAC Executive Committee.
You will receive a response within 10 working days to advise you whether your proposal has been approved. If you have any questions about accessing data, please email alspac-data@bristol.ac.uk.
Code availability
Code for data analyses is freely available on request.
References
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997).
Yeo, G. S. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20, 111–112 (1998).
Clément, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392, 398–401 (1998).
Gantz, I. et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 268, 15174–15179 (1993).
Mountjoy, K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, 1298–1308 (1994).
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163 (1999).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Haynes, W. G., Morgan, D. A., Djalali, A., Sivitz, W. I. & Mark, A. L. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33, 542–547 (1999).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 20, 113–114 (1998).
Farooqi, I. S. et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 106, 271–279 (2000).
Vaisse, C. et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Invest. 106, 253–262 (2000).
Hinney, A. et al. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J. Clin. Endocrinol. Metab. 91, 1761–1769 (2006).
Dempfle, A. et al. Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J. Med. Genet. 41, 795–800 (2004).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 (2003).
Collet, T.-H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 6, 1321–1329 (2017).
Stutzmann, F. et al. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 57, 2511–2518 (2008).
Turcot, V. et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 50, 26–41 (2018).
Gonçalves, J. P. L., Palmer, D. & Meldal, M. MC4R agonists: structural overview on antiobesity therapeutics. Trends Pharmacol. Sci. 39, 402–423 (2018).
Apovian, C. M. The obesity epidemic–understanding the disease and the treatment. N. Engl. J. Med. 374, 177–179 (2016).
Boyd, A. et al. Cohort profile: the ‘children of the 90s’–the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 42, 111–127 (2013).
Fraser, A. et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int. J. Epidemiol. 42, 97–110 (2012).
Cornish, R. P., Macleod, J., Boyd, A. & Tilling, K. Factors associated with participation over time in the Avon Longitudinal Study of Parents and Children: a study using linked education and primary care data. Int. J. Epidemiol. 50, 293–302 (2020).
Lotta, L. A. et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell 177, 597–607 (2019).
Greenfield, J. R. et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 360, 44–52 (2009).
Khera, A. V. et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 177, 587–596 (2019).
Krakoff, J. et al. Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes 57, 3267–3272 (2008).
Thearle, M. S. et al. Greater impact of melanocortin-4 receptor deficiency on rates of growth and risk of type 2 diabetes during childhood compared with adulthood in Pima Indians. Diabetes 61, 250–257 (2012).
Tunç, S. et al. Melanocortin-4 receptor gene mutations in a group of Turkish obese children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 9, 216–221 (2017).
Martinelli, C. E. et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J. Clin. Endocrinol. Metab. 96, E181–E188 (2011).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017).
Majithia, A. R. et al. Prospective functional classification of all possible missense variants in PPARG. Nat. Genet. 48, 1570–1575 (2016).
Chami, N., Preuss, M., Walker, R. W., Moscati, A. & Loos, R. J. F. The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS Med. 17, e1003196 (2020).
Baker, J. L., Olsen, L. W. & Sørensen, T. I. A. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 357, 2329–2337 (2007).
Bjerregaard, L. G. et al. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N. Engl. J. Med. 378, 1302–1312 (2018).
Richardson, T. G., Sanderson, E., Elsworth, B., Tilling, K. & Davey Smith, G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: Mendelian randomisation study. BMJ 369, m1203 (2020).
Northstone, K. et al. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res. 4, 51 (2019).
Howe, L. D. et al. Changes in ponderal index and body mass index across childhood and their associations with fat mass and cardiovascular risk factors at age 15. PLoS ONE 5, e15186 (2010).
Wade, K. H. et al. Assessing the causal role of body mass index on cardiovascular health in young adults. Circulation 138, 2187–2201 (2018).
Lang, R. M. et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463 (2005).
Leckie, G. & Charlton, C. runmlwin: a program to run the MLwiN multilevel modeling software from within Stata. J. Stat. Softw. 52, 1–40 (2013).
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We acknowledge the technical support of Tolulope Osunnuyi and the NIHR BRC-MRC BioRepository at Cambridge Biomedical Research Centre. We also thank staff members of the Wellcome Trust-MRC Institute of Metabolic Science Genomics and Transcriptomic core and the Genomics Core at the CRUK Cambridge Institute for their experimental support for next-generation sequencing. WES was obtained from ALSPAC (under proposal no. B2680) for comparison to the current study and we thank E. Robinson and B. Neale from the BROAD Institute for their contribution to exome sequencing. The UK MRC and Wellcome Trust (grant ref. 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. Genome-wide association data were generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. Mutational screening, sequencing and functional analyses were supported by MRC Metabolic Diseases Unit funding (MC_UU_00014/1). This publication is the work of all authors and K.H.W., N.J.T. and S.O. serve as guarantors for the contents of this paper. K.H.W. was supported by the Elizabeth Blackwell Institute for Health Research, University of Bristol and the Wellcome Trust Institutional Strategic Support Fund (204813/Z/16/Z). N.J.T. is a Wellcome Trust Investigator (202802/Z/16/Z), a workpackage lead in the Integrative Cancer Epidemiology Programme that is supported by a Cancer Research UK programme grant (C18281/A19169) and works within the University of Bristol National Institute for Health Research Biomedical Research Centre. D.A.H. and L.J.C. are supported by N.J.T.’s Wellcome Trust Investigator grant (202802/Z/16/Z) and work within the MRC Integrative Epidemiology Unit (MC_UU_00011). B.Y.H.L. is supported by a BBSRC Project Grant (BB/S017593/1). A. Melvin holds a PhD studentship supported jointly by the University of Cambridge Experimental Medicine Training Initiative programme in partnership with AstraZeneca. I.S.F. was supported by the Wellcome Trust (098497/Z/12/Z), the NIHR Cambridge Biomedical Research Centre, the Botnar Fondation and the Bernard Wolfe Health Neuroscience Endowment and a Wellcome Developing Concept Fund award (with J.M.). S.O.R. and G.S.H.Y. is supported by the MRC Metabolic Disease Unit (MC_UU_00014/1) and S.O.R. by a Wellcome Trust Investigator award (WT 095515/Z/11/Z) and National Institute for Health Research Cambridge Biomedical Research Centre. The Wellcome-MRC Institute of Metabolic Science Genomics and transcriptomics core facility is supported by the Medical Research Council (MC_UU_00014/5) and the Wellcome Trust (208363/Z/17/Z).
Author information
Authors and Affiliations
Contributions
K.H.W., B.Y.H.L., A. Melvin, G.S.H.Y., N.J.T. and S.O.R. designed the study. C.Z., K.R. and K.D. conducted the genomic sequencing. J.M. and I.S.F. contributed to the design of in vitro assays. W.P., J.H.C., K.L., K.D., J.M. and A.W. planned and performed the in vitro studies. B.Y.H.L., A. Melvin and A. Mörseburg conducted bioinformatic analysis and analyzed the data from in vitro experiments. K.H.W. conducted the analysis of phenotype data in the ALSPAC cohort. L.J.C. critically reviewed the analysis in ALSPAC and the manuscript. D.A.H., K.N. and S.N. were involved in early conversations on the project and provided access to phenotypic, genotypic and exome data. K.H.W., B.Y.H.L., A. Melvin, G.S.H.Y., N.J.T. and S.O.R. wrote the manuscript and it was reviewed by all authors.
Corresponding authors
Ethics declarations
Competing interests
S.O.R. has undertaken remunerated consultancy work for Pfizer, AstraZeneca, GSK and ERX Pharmaceuticals. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Medicine thanks Anke Hinney and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Jennifer Sargent was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Differences in body mass index in those sequenced for MC4R LoF mutations and those not sequenced from the rest of ALSPAC.
Estimates reflect the mean difference (95% CI) in BMI (kg/m2) comparing those sequenced for MC4R LoF mutations to those not sequenced, obtained from two-sided t-tests (p-values were not corrected for multiple comparisons). ALSPAC = Avon Longitudinal Study of Parents and Children; BMI = body mass index; CI = confidence interval; LoF = loss of function.
Extended Data Fig. 2 β-arrestin-2 functional classification of MC4R variants.
Colors represent cLoF (purple), pLoF (light brown), WT-like (green) and GoF (black). β-arrestin-2 coupling activity of MC4R mutations were characterised using a protein-protein interaction assay. a, Dose response curves of MC4R mutants upon activation by NDP-MSH grouped by cLoF, pLoF, WT-like and GoF compared wild-type MC4R. Means + /- S.E.M. are shown (N = 3–8 independent experiments); and b, The relative EC50 (fold WT response, left panel) and Emax (% WT response right panel) were determined for each mutants and are presented in mean and 95% CI. * indicates p < 0.05 by paired t-test (p-values were two-sided and uncorrected for multiple comparisons). CI = confidence interval; cLoF = complete loss of function; GoF = gain of function; N.D. = not determined; NDP-αMSH = [Nle4,D-Phe7]-α-melanocyte-stimulating hormone; pLoF = partial loss of function; WT-like = wild-type like.
Extended Data Fig. 3 Mean weight across time with MC4R LoF of cAMP accumulation.
Mean weight +/− 95% CI at different ages (sample sizes across all ages presented in Supplementary Table 4) with MC4R LoF of cAMP (carriers of pLoF and cLoF) and the reference group (that is, non-LoF carriers – combining individuals with synonymous, common variations or no LoF mutations and individuals with WT-like and GoF mutations). Figures only show results where all mutational groups (that is, WT-like, GoF, pLoF and cLoF mutations) were represented by at least one individual at all time points between birth and 24 years. CI = confidence interval; cLoF = complete loss of function; GoF = gain of function; LoF = loss of function; pLoF = partial loss of function; WT = wild-type.
Extended Data Fig. 4 Mean height across time with MC4R LoF of cAMP accumulation.
Mean height +/− 95% CI at different ages (sample sizes across ages presented in Supplementary Table 4) with MC4R LoF of cAMP (carriers of pLoF and cLoF) and the reference group (that is, non-LoF carriers – combining individuals with synonymous, common variations or no LoF mutations and individuals with WT-like and GoF mutations). Figures only show results where all mutational groups (that is, WT-like, GoF, pLoF and cLoF mutations) were represented by at least one individual at all time points between birth and 24 years. CI = confidence interval; cLoF = complete loss of function; GoF = gain of function; LoF = loss of function; pLoF = partial loss of function; WT = wild-type.
Extended Data Fig. 5 Association between MC4R LoF of cAMP accumulation and arterial BP across age in a model adjusting only for sex and a model adjusted for sex and BMI at the same age.
Estimates represent the change (mmHg) in SBP or DBP (top and bottom panel, respectively) in carriers (that is, pLoF or cLoF) vs. non-LoF carriers (that is, individuals with synonymous, common variations or no LoF mutations and individuals with WT-like and GoF mutations) of MC4R LoF mutations, obtained from linear regression (p-values presented are two-sided and not corrected for multiple comparisons). BMI = body mass index; BP = blood pressure; CI = confidence interval; cLoF = complete loss of function; DBP = diastolic blood pressure; GoF = gain of function; LoF = loss of function; pLoF = partial loss of function; SBP = systolic blood pressure; WT-like = wild-type like.
Extended Data Fig. 6 Association of MC4R LoF of cAMP accumulation with both central BP and LVMI at age 18 years in a model adjusting only for sex and a model adjusted for sex and BMI at the same age.
Estimates represent the change in central DBP (mmHg), central SBP (mmHg) and LVMI (g/m2.7) in carriers (that is, pLoF or cLoF) vs. non-LoF carriers (that is, individuals with synonymous, common variations or no LoF mutations and individuals with WT-like and GoF mutations) of MC4R LoF mutations, obtained from linear regression (p-values presented are two-sided and not corrected for multiple comparisons). BMI = body mass index; CI = confidence interval; cLoF = complete loss of function; DBP = diastolic blood pressure; GoF = gain of function; LoF = loss of function; LVMI = left ventricular mass index; pLoF = partial loss of function; SBP = systolic blood pressure; WT-like = wild-type like.
Extended Data Fig. 7 Association between MC4R LoF of cAMP accumulation with weight and height trajectories between birth and 18 years using linear spline multi-level models.
Values for the reference group (that is, all individuals with synonymous, common variations or no LoF mutations and individuals with WT-like and GoF mutations) and LoF mutations (that is, combining pLoF and cLoF mutations) are depicted in light and dark blue, respectively (N = 5716). Effect estimates and confidence intervals of these associations are presented in Supplementary Table 11 and Supplementary Table 13 for weight and height, respectively, and were generated using linear spline multi-level models. cLoF = complete loss of function; GoF = gain of function; LoF = loss of function; pLoF = partial loss of function; WT-like = wild-type like.
Extended Data Fig. 8 Mean BMI across time with MC4R LoF of β-arrestin-2 coupling.
Mean BMI +/− 95% CI at different ages (sample sizes across all ages presented in Supplementary Table 14) with MC4R LoF of β-arrestin-2 (carriers of pLoF and cLoF) and the reference group (that is, non-LoF carriers – combining individuals with synonymous, common variations or no LoF mutations and individuals with WT-like and GoF mutations). Figures only show results where all mutational groups (that is, WT-like, GoF, pLoF and cLoF mutations) were represented by at least one individual at all time points between birth and 24 years. CI = confidence interval; cLoF = complete loss of function; GoF = gain of function; LoF = loss of function; pLoF = partial loss of function; WT = wild-type.
Extended Data Fig. 9 Mean weight and height across time with MC4R LoF of β-arrestin-2 coupling.
Mean weight and height +/− 95% CI at different ages (sample sizes across all ages presented in Supplementary Table 14) with MC4R LoF of β-arrestin-2 (carriers of pLoF and cLoF) and the reference group (that is, non-LoF carriers – combining individuals with synonymous, common variations or no LoF mutations and individuals with WT-like and GoF mutations). Figures only show results where all mutational groups (that is, WT-like, GoF, pLoF and cLoF mutations) were represented by at least one individual at all time points between birth and 24 years. CI = confidence interval; cLoF = complete loss of function; GoF = gain of function; LoF = loss of function; pLoF = partial loss of function; WT = wild-type.
Extended Data Fig. 10 Identification of rare MC4R variants by pooled sequencing.
Pooled MC4R sequencing workflow for DNA samples from the ALSPAC cohort; b, Agarose gel showing a 1130 bp PCR product using MC4R exon primers (Supplementary Table 1), PCR products from 2 independent DNA samples are shown; c, A plot showing the sequencing coverage of MC4R coding region from a representative pool. The average per-base sequencing depth for all pools was 43,654 ± 356-fold; d, The percentage of true positive and false positive calls binned by variant allele frequency (VAF) detected in pools; e, Receiver operating curve analysis of VAF and call accuracy; f, Comparison of participants included in the current study and another whole-exome sequencing (WES) study (with ALSPAC project number: B2680); g, Comparison of non-synonymous variant carriages between this study and WES study. Of 40 mutational carriages found in Cambridge, 15 were found in both studies. 25 carriers found in the Cambridge study were not part of the WES study and 4 carriers found only in the WES study were not part of the Cambridge study. *rare = minor allele frequency < 0.01%, both p.V103I and p.I251L were above 0.01% and excluded in the analyses. ALSPAC = Avon Longitudinal Study of Parents and Children; AUC = area under the curve; HTS = high-throughput sequencing; WES = whole-exome sequencing.
Supplementary information
Supplementary Information
Supplementary Tables 1–20.
Rights and permissions
About this article
Cite this article
Wade, K.H., Lam, B.Y.H., Melvin, A. et al. Loss-of-function mutations in the melanocortin 4 receptor in a UK birth cohort. Nat Med 27, 1088–1096 (2021). https://doi.org/10.1038/s41591-021-01349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01349-y
This article is cited by
-
The melanocortin action is biased toward protection from weight loss in mice
Nature Communications (2023)
-
Congenital leptin and leptin receptor deficiencies in nine new families: identification of six novel variants and review of literature
Molecular Genetics and Genomics (2023)
-
Weighted burden analysis in 200,000 exome-sequenced subjects characterises rare variant effects on BMI
International Journal of Obesity (2022)
-
The genetics of obesity: from discovery to biology
Nature Reviews Genetics (2022)
-
The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits
Nature Reviews Endocrinology (2022)