Abstract
Objective
To compare Orsiro biodegradable-polymer sirolimus-eluting stent (Orsiro BP-SES) with durable-polymer everolimus-eluting stent (DP-EES) regarding target lesion failure (TLF) after rotational atherectomy (RA), with a focus on small stents (diameter ≤ 3 mm) where Orsiro BP-SES has 60 µm strut thickness, while DP-EES remains with 81 µm strut thickness.
Background
New-generation drug-eluting stent (DES) is superior to early-generation DES in all percutaneous coronary intervention (PCI) settings including RA. Recently, the Orsiro BP-SES was superior to a DP-EES in an all comer’s population.
Methods
Among patients who underwent RA at a single center, 121 were treated with Orsiro BP-SES and 164 with DP-EES (Promus and Xience). Those treated with other stent types, presenting with acute myocardial infarction or had a chronic total occlusion were excluded. Incidence of TLF was assessed.
Results
After 2 years, the TLF rate in Orsiro BP-SES and DP-EES groups was 10% and 18%, respectively (adjusted HR 0.55, 95%CI 0.26–1.16, p = 0.115). The rate of TLF was significantly lower in small Orsiro BP-SES with ultra-thin struts as compared to DP-EES with the same diameters (adjusted HR 0.19, 95% CI 0.04–0.87, p = 0.032), driven by lower rates of clinically driven target lesion revascularization (log-rank p = 0.022). Age (p = 0.035), total stent length (p = 0.007) and diabetes mellitus (p = 0.011) emerged as independent predictors of TLF in the whole population.
Conclusion
In the whole cohort, Orsiro BP-SES and DP-EES had comparable rates of long-term TLF after RA. In the small stent subgroup, the Orsiro BP-SES with ultra-thin struts showed significant lower rate of TLF at 2 years.
Graphic abstract




Similar content being viewed by others
Data availability
The deidentified participant data will be shared on a request basis. Please directly contact the corresponding author to request data sharing.
Code availability
Data analysis was performed using SPSS V.24.0 (IBM Corp., New York, USA).
Abbreviations
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass graft
- CAC:
-
Coronary artery calcification
- CTO:
-
Chronic total occlusion
- DES:
-
Drug-eluting stent
- DP-EES:
-
Durable-polymer everolimus-eluting stent
- GFR:
-
Glomerular filtration rate
- LVEF:
-
Left ventricular ejection fraction
- MACE:
-
Major adverse cardiac events
- MI:
-
Myocardial infarction
- Orsiro BP-SES:
-
Orsiro biodegradable-polymer sirolimus-eluting stent
- PCI:
-
Percutaneous coronary intervention
- RA:
-
Rotational atherectomy
- ST:
-
Stent thrombosis
- TLF:
-
Target lesion failure
- TLR:
-
Target-lesion revascularization
- TV-MI:
-
Target vessel myocardial infarction
- UFH:
-
Unfractionated heparin
References
Genereux P, Madhavan MV, Mintz GS et al (2014) Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) and ACUITY (acute catheterization and urgent intervention triage strategy) trials. J Am Coll Cardiol 63(18):1845–1854
Takebayashi H, Kobayashi Y, Mintz GS et al (2005) Intravascular ultrasound assessment of lesions with target vessel failure after sirolimus-eluting stent implantation. Am J Cardiol 95(4):498–502. https://doi.org/10.1016/j.amjcard.2004.10.020
Kobayashi Y, Okura H, Kume T et al (2014) Impact of target lesion coronary calcification on stent expansion. Circ J 78(9):2209–2214. https://doi.org/10.1253/circj.cj-14-0108
Madhavan MV, Tarigopula M, Mintz GS et al (2014) Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol 63(17):1703–1714. https://doi.org/10.1016/j.jacc.2014.01.017
Barbato E, Carrie D, Dardas P et al (2015) European expert consensus on rotational atherectomy. EuroIntervention 11(1):30–36. https://doi.org/10.4244/EIJV11I1A6
Barbato E, Shlofmitz E, Milkas A et al (2017) State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses—from debulking to plaque modification, a 40-year-long journey. EuroIntervention 13(6):696–705. https://doi.org/10.4244/EIJ-D-17-00473
Dill T, Dietz U, Hamm CW et al (2000) A randomized comparison of balloon angioplasty versus rotational atherectomy in complex coronary lesions (COBRA study). Eur Heart J 21(21):1759–1766. https://doi.org/10.1053/euhj.2000.2242
Moussa I, Di Mario C, Moses J et al (1997) Coronary stenting after rotational atherectomy in calcified and complex lesions. Angiographic and clinical follow-up results. Circulation 96(1):128–136. https://doi.org/10.1161/01.cir.96.1.128
Allali A, Holy EW, Sulimov DS et al (2018) Long-term clinical outcome of early generation versus new-generation drug-eluting stents in 481 patients undergoing rotational atherectomy: a retrospective analysis. Cardiology and therapy 7(1):89–99. https://doi.org/10.1007/s40119-017-0101-y
Jensen LO, Thayssen P, Christiansen EH et al (2016) Safety and efficacy of everolimus- versus sirolimus-eluting stents: 5-year results from SORT OUT IV. J Am Coll Cardiol 67(7):751–762. https://doi.org/10.1016/j.jacc.2015.11.051
Byrne RA, Stone GW, Ormiston J et al (2017) Coronary balloon angioplasty, stents, and scaffolds. Lancet 390(10096):781–792. https://doi.org/10.1016/S0140-6736(17)31927-X
Joner M, Finn AV, Farb A et al (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48(1):193–202. https://doi.org/10.1016/j.jacc.2006.03.042
Saito S, Toelg R, Witzenbichler B et al (2019) BIOFLOW-IV, a randomised, intercontinental, multicentre study to assess the safety and effectiveness of the Orsiro sirolimus-eluting stent in the treatment of subjects with de novo coronary artery lesions: primary outcome target vessel failure at 12 months. EuroIntervention 15(11):e1006–e1013. https://doi.org/10.4244/EIJ-D-18-01214
Kandzari DE, Mauri L, Koolen JJ et al (2017) Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet 390(10105):1843–1852. https://doi.org/10.1016/S0140-6736(17)32249-3
Windecker S, Haude M, Neumann FJ et al (2015) Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv 8(2):e001441. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001441
Buiten RA, Ploumen EH, Zocca P et al (2019) Outcomes in patients treated with thin-strut, very thin-strut, or ultrathin-strut drug-eluting stents in small coronary vessels: a prespecified analysis of the randomized BIO-RESORT trial. JAMA cardiology 4(7):659–669. https://doi.org/10.1001/jamacardio.2019.1776
Kereiakes DJ, Meredith IT, Windecker S et al (2015) Efficacy and safety of a novel bioabsorbable polymer-coated everolimus-eluting coronary stent: the EVOLVE II randomized trial. Circ Cardiovasc Interv. https://doi.org/10.1161/CIRCINTERVENTIONS.114.002372
Abdel-Wahab M, Toelg R, Byrne RA et al (2018) High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv 11(10):e007415. https://doi.org/10.1161/CIRCINTERVENTIONS.118.007415
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. Circulation 126(16):2020–2035. https://doi.org/10.1161/CIR.0b013e31826e1058
Garcia-Garcia HM, McFadden EP, Farb A et al (2018) Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Eur Heart J 39(23):2192–2207. https://doi.org/10.1093/eurheartj/ehy223
Iglesias JF, Roffi M, Degrauwe S et al (2017) Orsiro cobalt-chromium sirolimus-eluting stent: present and future perspectives. Expert Rev Med Devices 14(10):773–788. https://doi.org/10.1080/17434440.2017.1378091
Kawamoto H, Panoulas VF, Sato K et al (2015) Impact of strut width in periprocedural myocardial infarction: a propensity-matched comparison between bioresorbable scaffolds and the first-generation sirolimus-eluting stent. JACC Cardiovasc Interv 8(7):900–909. https://doi.org/10.1016/j.jcin.2015.02.011
Hausleiter J, Kastrati A, Mehilli J et al (2003) Impact of lesion complexity on the capacity of a trial to detect differences in stent performance: results from the ISAR-STEREO trial. Am Heart J 146(5):882–886. https://doi.org/10.1016/S0002-8703(03)00435-6
Torii S, Jinnouchi H, Sakamoto A et al (2019) Vascular responses to coronary calcification following implantation of newer-generation drug-eluting stents in humans: impact on healing. Eur Heart J. https://doi.org/10.1093/eurheartj/ehz850
Liu Y, Liu Y, Zheng Y et al (2019) Catheter thermal energy generation and temperature in rotational atherectomy. Med Eng Phys 70:29–38. https://doi.org/10.1016/j.medengphy.2019.06.014
Abdel-Wahab M, Richardt G, Joachim Buttner H et al (2013) High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (rotational atherectomy prior to taxus stent treatment for complex native coronary artery disease) trial. JACC Cardiovasc Interv 6(1):10–19. https://doi.org/10.1016/j.jcin.2012.07.017
Pilgrim T, Heg D, Roffi M et al (2014) Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet 384(9960):2111–2122. https://doi.org/10.1016/S0140-6736(14)61038-2
Cassese S, Ndrepepa G, Byrne RA et al (2018) Outcomes of patients treated with ultrathin-strut biodegradable polymer sirolimus-eluting stents versus fluoropolymer-based everolimus-eluting stents: a meta-analysis of randomised trials. EuroIntervention 14(2):224–231. https://doi.org/10.4244/EIJ-D-18-00024
Pilgrim T, Piccolo R, Heg D et al (2018) Ultrathin-strut, biodegradable-polymer, sirolimus-eluting stents versus thin-strut, durable-polymer, everolimus-eluting stents for percutaneous coronary revascularisation: 5-year outcomes of the BIOSCIENCE randomised trial. Lancet 392(10149):737–746. https://doi.org/10.1016/S0140-6736(18)31715-X
Lefevre T, Haude M, Neumann FJ et al (2018) Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: 5-year outcomes of the randomized BIOFLOW-II trial. JACC Cardiovasc Interv 11(10):995–1002. https://doi.org/10.1016/j.jcin.2018.04.014
Teeuwen K, van der Schaaf RJ, Adriaenssens T et al (2017) Randomized multicenter trial investigating angiographic outcomes of hybrid sirolimus-eluting stents with biodegradable polymer compared with everolimus-eluting stents with durable polymer in chronic total occlusions: the PRISON IV trial. JACC Cardiovasc Interv 10(2):133–143. https://doi.org/10.1016/j.jcin.2016.10.017
Iglesias JF, Muller O, Heg D et al (2019) Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet 394(10205):1243–1253. https://doi.org/10.1016/S0140-6736(19)31877-X
Pache J, Kastrati A, Mehilli J et al (2003) Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol 41(8):1283–1288. https://doi.org/10.1016/s0735-1097(03)00119-0
Kolandaivelu K, Swaminathan R, Gibson WJ et al (2011) Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 123(13):1400–1409. https://doi.org/10.1161/CIRCULATIONAHA.110.003210
Elezi S, Dibra A, Mehilli J et al (2006) Vessel size and outcome after coronary drug-eluting stent placement: results from a large cohort of patients treated with sirolimus- or paclitaxel-eluting stents. J Am Coll Cardiol 48(7):1304–1309. https://doi.org/10.1016/j.jacc.2006.05.068
Cassese S, Byrne RA, Tada T et al (2014) Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 100(2):153–159. https://doi.org/10.1136/heartjnl-2013-304933
Kastrati A, Mehilli J, Dirschinger J et al (2001) Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 103(23):2816–2821. https://doi.org/10.1161/01.cir.103.23.2816
Bangalore S, Toklu B, Patel N et al (2018) Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation 138(20):2216–2226. https://doi.org/10.1161/CIRCULATIONAHA.118.034456
Iglesias JF, Heg D, Roffi M et al (2019) Long-term effect of ultrathin-strut versus thin-strut drug-eluting stents in patients with small vessel coronary artery disease undergoing percutaneous coronary intervention: a subgroup analysis of the BIOSCIENCE randomized trial. Circ Cardiovasc Interv 12(8):e008024. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008024
Serruys PW, Farooq V, Kalesan B et al (2013) Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (limus eluted from a durable versus erodable stent coating) randomized, non-inferiority trial. JACC Cardiovasc Interv 6(8):777–789. https://doi.org/10.1016/j.jcin.2013.04.011
Kufner S, Byrne RA, Valeskini M et al (2016) Five-year outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: final results from the ISAR-TEST 4 randomised trial. EuroIntervention 11(12):1372–1379. https://doi.org/10.4244/EIJY14M11_02
El-Hayek G, Bangalore S, Casso Dominguez A et al (2017) Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc Interv 10(5):462–473. https://doi.org/10.1016/j.jcin.2016.12.002
Dan K, Garcia-Garcia HM, Kolm P et al (2020) Comparison of ultrathin, bioresorbable-polymer sirolimus-eluting stents and thin, durable-polymer everolimus-eluting stents in calcified or small vessel lesions. Circ Cardiovasc Interv 13(9):e009189. https://doi.org/10.1161/CIRCINTERVENTIONS.120.009189
Toelg R, Slagboom T, Waltenberger J et al (2020) Individual patient data analysis of the BIOFLOW study program comparing safety and efficacy of a bioresorbable polymer sirolimus eluting stent to a durable polymer everolimus eluting stent. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.29254
Mintz GS, Guagliumi G (2017) Intravascular imaging in coronary artery disease. Lancet 390(10096):793–809. https://doi.org/10.1016/S0140-6736(17)31957-8
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Hemetsberger received speaker’s honoraria from Boston Scientific. Dr. Richardt received an Institutional Research Grant from Boston Scientific. Dr. Allali is a proctor for Boston Scientific. The other authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. The present study was approved by the Ethics Committee of Schleswig–Holstein Medical Syndicate. Reference Number: 168-11.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Supplementary Information
Below is the link to the electronic supplementary material.
392_2021_1852_MOESM1_ESM.tif
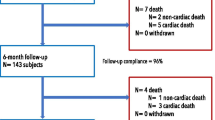
Online resource Fig. 1 Study Flow-chart MI, myocardial infarction; CTO, chronic total occlusion; Orsiro BP-SES, Orsiro biodegradable-polymer sirolimus-eluting stent; DP-EES, durable-polymer evorolimus eluting stent
Rights and permissions
About this article
Cite this article
Mankerious, N., Hemetsberger, R., Traboulsi, H. et al. Outcomes of patients treated with a biodegradable-polymer sirolimus-eluting stent versus durable-polymer everolimus-eluting stents after rotational atherectomy. Clin Res Cardiol 110, 1574–1585 (2021). https://doi.org/10.1007/s00392-021-01852-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01852-9