Abstract
Background
Coronary bypass artery grafting (CABG) has a higher procedural risk of stroke than percutaneous coronary intervention (PCI), but may offer better long-term survival. The optimal revascularization strategy for patients with prior cerebrovascular disease (CEVD) remains unclear.
Methods and results
The SYNTAXES study assessed the vital status out to 10 year of patients with three-vessel disease and/or left main coronary artery disease enrolled in the SYNTAX trial. The relative efficacy of PCI vs. CABG in terms of 10 year all-cause death was assessed according to prior CEVD. The primary endpoint was 10 year all-cause death. The status of prior CEVD was available in 1791 (99.5%) patients, of whom 253 patients had prior CEVD. Patients with prior CEVD were older and had more comorbidities (medically treated diabetes, insulin-dependent diabetes, metabolic syndrome, peripheral vascular disease, chronic obstructive pulmonary disease, impaired renal function, and congestive heart failure), compared with those without prior CEVD. Prior CEVD was an independent predictor of 10 year all-cause death (adjusted HR: 1.35; 95% CI: 1.04–1.73; p = 0.021). Patients with prior CEVD had a significantly higher risk of 10 year all-cause death (41.1 vs. 24.1%; HR: 1.92; 95% CI: 1.54–2.40; p < 0.001). The risk of 10 year all-cause death was similar between patients receiving PCI or CABG irrespective of the presence of prior CEVD (p-interaction = 0.624).
Conclusion
Prior CEVD was associated with a significantly increased risk of 10 year all-cause death which was similar in patients treated with PCI or CABG. These results do not support preferential referral for PCI rather than CABG in patients with prior CEVD.
Trial registration: SYNTAX: ClinicalTrials.gov reference: NCT00114972. SYNTAX Extended Survival: ClinicalTrials.gov reference: NCT03417050.
Graphic abstract

Similar content being viewed by others
Introduction
The relationship between cerebrovascular disease (CEVD) and coronary artery disease (CAD) has been extensively investigated with numerous studies confirming that CEVD shares common vascular risk factors with CAD [1, 2]. Moreover, the presence of CEVD is associated with worse clinical outcomes after coronary revascularization, and has been reported to be an independent risk factor of long-term mortality in patients with CAD [3,4,5].
Randomized controlled trials (RCTs) have consistently shown that coronary revascularization by coronary artery bypass grafting (CABG) as opposed to percutaneous coronary intervention (PCI) is associated with an increased risk of stroke [6,7,8]. In contemporary clinical practice, patients with prior CEVD are often referred for PCI instead of CABG due to concerns from patients and cardiovascular physicians of a higher rate of perioperative stroke and cognitive decline after CABG. Of note, patients with prior CEVD are more likely to have more extensive CAD than those without CEVD, which can lead to poorer clinical outcomes after PCI [3,4,5, 9]. Therefore, determining the optimal method of revascularization for patients with prior CEVD remains challenging.
To date, there are no data evaluating the impact of prior CVED on long-term (up to 10 years) mortality after revascularization, especially in patients with de novo three-vessel (3VD) and/or left main coronary artery disease (LMCAD). The SYNTAX Extended Survival (SYNTAXES) study established unique 10 year all-cause death in 94% all-comers patients with de novo 3VD and/or LMCAD who were originally randomized to CABG or PCI in the SYNTAX trial [10]. We therefore aim to evaluate the relative benefit of PCI versus CABG in terms of all-cause death at 10 years according to prior CEVD in the SYNTAXES study.
Methods
Study design and population
The SYNTAX study design and the primary and final 5 year results of the trial have been published previously [11,12,13]. In brief, the trial was a prospective, international, multicenter, RCT conducted at 85 centers in Europe and the United States between March 2005 and April 2007. Based on clinical judgment and the consensus of the Heart Team consisting of a cardiothoracic surgeon and an interventional cardiologist and supported by the study coordinator at each center, all-comers patients with de novo 3VD and/or LMCAD in whom clinical equipoise in terms of revascularization strategy between CABG and PCI was assumed, were enrolled and randomized in a 1:1 fashion to either CABG (n = 897) or PCI (n = 903) with TAXUS Express paclitaxel-drug eluting stents (PES) (Boston Scientific Corporation, Marlborough, MA, USA). The SYNTAX trial (NCT00114972) completed patient follow-up up to 5 years [13]. The SYNTAXES study (NCT03417050) was an investigator-driven initiative that extended follow-up and aimed to evaluate vital status up to 10 years [10]. The extended follow-up was funded by the German Heart Research Foundation (GHF; Frankfurt am Main, Germany). Follow-up was performed in accordance with local regulations of each participating site and complied with the declaration of Helsinki. Informed consent to assess vital status up to 10 year of follow-up was waived by the medical ethical committee.
Definition of prior CEVD
Prior CEVD was defined as prior stroke, transient ischemic attack (TIA), or carotid artery disease (carotid stent, endarterectomy, known carotid stenosis or bruit without revascularization, or other), which is consistent with a previous report of the EXCEL trial [14]. The presence of prior CEVD was assessed in every patient before randomization by the investigators and collected on the electronic case report form.
Study endpoints
The pre-specified primary endpoint of the SYNTAXES study was all-cause death at 10 years. The pre-specified secondary endpoint was all-cause death at maximum follow-up. Vital status was confirmed by electronic healthcare record review and national death registry.
Statistical analyses
All the analyses were performed according to intention to treat principle. The cumulative incidence of clinical adverse events up to 10 years was assessed using the Kaplan–Meier method and compared using the log-rank test. Hazard ratio (HR) with 95% confidence interval (CI) was assessed by a Cox proportional regression model. Multivariate analysis was performed to evaluate whether prior CEVD was an independent predictor of all-cause death at 10 year or the maximum follow-up. The Cox proportional hazards regression model included the following covariates: age, gender, body mass index, hypertension, dyslipidemia, diabetes mellitus, current smoking, peripheral vascular disease, Chronic Obstructive Pulmonary Disease (COPD), impaired renal function (defined as a calculated creatinine clearance < 60 ml/min using the Cockcroft–Gault equation), prior myocardial infarction, the anatomical SYNTAX score and randomized strategy (CABG or PCI). Unfortunately, the relatively small numbers of the specific components of CEVD precluded analysis of the effect of revascularization by type of CEVD.
Continuous variables are reported as mean ± standard deviations (SD) or median and interquartile range (IQR), and were compared using Student’s t tests or Mann–Whitney U test, respectively. Categorical variables are reported as percentages and numbers and were compared using Chi-square or Fisher’s exact test as appropriate. All tests are two-sided and a p value of < 0.05 was considered to be statistically significant. All analyses were performed using SPSS Statistics, version 25 (IBM Corp., Armonk, 281NY, USA).
Results
Study population
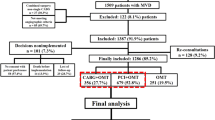
In the SYNTAX trial, a total of 1800 patients were randomly assigned to undergo PCI with paclitaxel eluting stents (n = 903) or CABG (n = 897). The status of prior CEVD was available in 1791 (99.5%) patients who made up the cohort for the present analysis. Among them, 253 (14.1%) patients had prior CEVD (78 patients had prior stroke, 84 patients had prior TIA, and 148 had prior carotid artery disease) (Fig. 1). Vital status at 10 year follow-up was complete in 839 (93%) patients in the PCI group and 841 (94%) patients in the CABG group.
Outcomes according to prior CEVD
Baseline characteristics according to prior CEVD are shown in Table 1. Patients with prior CEVD were older, had more comorbidities (medically treated diabetes, insulin-dependent diabetes, metabolic syndrome, peripheral vascular disease, chronic obstructive pulmonary disease, impaired renal function, and congestive heart failure), and had a higher EuroSCORE and Parsonnet SCORE, compared with those without prior CEVD. Patients with prior CEVD had more lesions treated compared with those without prior CEVD.
The median duration of follow-up was 11.2 years (IQR: 7.7–12.1) overall and 11.9 years (IQR: 11.2–12.4) in survivors. When compared to those without prior CEVD, patients with prior CEVD had a significantly higher risk of all-cause death at 10 years (41.1 vs. 24.1%; HR: 1.92; 95% CI: 1.54–2.40; p < 0.001) and at maximum follow-up of 12.6 years (53.8 vs. 32.5%; HR: 1.99; 95% CI: 1.62–2.43; p < 0.001) (Fig. 2a, Online Fig. S1A, Table 2).
By multivariate analysis, prior CEVD was an independent predictor of all-cause death at 10 years (adjusted HR: 1.35; 95% CI: 1.04–1.73; p = 0.021) and at maximum follow-up of 12.6 years (adjusted HR: 1.45; 95% CI: 1.16–1.82; p = 0.001) (Online Tables S1 and S2).
Clinical outcomes according to revascularization strategy
Among patients with prior CEVD, 119 and 134 patients were randomly assigned to PCI and CABG, respectively. Among 1538 patients without prior CEVD, 782 and 756 patients were randomized to PCI and CABG, respectively (Fig. 1).
Baseline clinical and procedural characteristics according to prior CEVD and revascularization strategies are shown in Table 3. By randomization, baseline clinical and procedural characteristics were largely well balanced between PCI and CABG in patients with and without prior CEVD.
Compared with those without prior CEVD, the risk of 10-year all-cause death was higher in patients with prior CEVD both in the PCI arm (46.0 vs. 25.9%; HR: 2.06; 95% CI: 1.52–2.79; p < 0.001) and in the CABG arm (36.8 vs. 22.2%; HR: 1.83; 95% CI: 1.32–2.53; p < 0.001) (Fig. 2b, c), and these differences remained significant at maximum follow-up of 12.6 years for PCI (53.8 vs. 35.7%; HR: 1.93; 95% CI: 1.45–2.57; p < 0.001) and CABG (53.2 vs. 29.2%; HR: 2.09; 95% CI: 1.57–2.77; p < 0.001) (Online Fig. S1b, c). However, the risk of all-cause death at 10 years was similar between PCI and CABG irrespective of the presence of prior CEVD (P-interaction = 0.624) (Table 4).
Clinical outcomes according to complexity of coronary artery disease (3VD or LMCAD)
The limited number of events precluded a subgroup analysis according to SYTNTAX score; we performed the analysis according to 3VD or LMCAD. Results demonstrated that rates of all-cause death at 10 years and maximum follow-up were numerically higher after PCI than after CABG but not significantly different in both 3VD and LMCAD patients with prior CEVD (Online Fig. S2).
Discussion
The SYNTAXES study is the first study to investigate 10year survival after PCI with drug eluting stents versus CABG in patients with de novo3VD and/or LMCAD. The present analysis is the first study to evaluate the potential relative benefit of PCI versus CABG in terms of all-cause death at 10 years according to prior CEVD in stable patients with complex CAD. The main findings of the present study can be summarized as follows: (1) prior CEVD (14.1%) was common among patients with de novo 3VD and/or LMCAD and they had more comorbidities and more extensive CAD compared with those without CEVD; (2) prior CEVD was associated with a significantly increased risk of all-cause death at 10 years; (3) the relative effects of PCI versus CABG on 10 year all-cause death were similar, irrespective of whether patients had prior CEVD or not.
Patients with CAD often have prior CEVD, which itself is associated with a higher prevalence of CAD [1, 2, 15]. Numerous studies have demonstrated that CAD patients with prior CEVD are more likely to have a diffuse, complex and higher disease burden and multiple comorbidities [3,4,5]. Patients with prior CEVD therefore represent a high risk population and are often excluded from coronary revascularization trials. However, with advances in PCI and CABG techniques, more and more patients with prior CEVD are undergoing revascularization in contemporary practice. In our study, 14.1% of patients who underwent coronary revascularization had a prior history of CEVD, which is comparable to the 12.3% observed in the EXCEL (Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trial [14].
Prior CEVD has been shown to be associated with worse clinical outcomes after coronary revascularization [3,4,5, 9, 16]. Indeed, we found that prior CEVD was associated with a significantly increased risk of all-cause death at 10 years in both the PCI and CABG arms. These poorer outcomes may most likely be due to the advanced age and presence of a greater number of comorbidities (peripheral vascular disease, chronic obstructive pulmonary disease, impaired renal function) and cardiac risk factors (diabetes, metabolic syndrome) in the CEVD patient cohort (Table 1), some of which were also found to be independent predictors of 10 year all-cause mortality. These observations were further validated by the fact that history of prior CEVD remained an independent predictor of all-cause death at 10 years and at maximum follow-up (12.6 years)even after multivariate adjustment for important clinical confounders (Online Tables S1 and S2).
The optimal revascularization strategy for complex CAD patients with prior CEVD remains unclear. Stroke is one of the most devastating complications after coronary revascularization, leading to a higher risk of mortality and permanent disability [17]. Most previous studies demonstrated that CABG carried a higher rate of stroke, especially in the periprocedural period [6,7,8, 18]. Hence, in clinical practice, patients with prior CEVD are often referred for PCI instead of CABG. However, recent studies have shown that CABG only increased the risk of perioperative stroke, while the rate of long-term stroke was comparable between PCI and CABG [7, 18,19,20,21]. Moreover, as aforementioned, patients with prior CEVD, who have complex and diffuse CAD and multiple comorbidities, and who undergo PCI may experience increased rates of recurrent cerebrovascular events, myocardial infarction, and death9, [16, 22]. It is important to balance the risk of stroke, which represents the major adverse event of CABG, against the risk of other adverse events such as repeat revascularization, myocardial infarction and death, when determining the optimal revascularization modality between CABG and PCI in patients with prior CEVD [23, 24]. Hence, whether high-risk patients with prior CEVD would benefit from PCI rather than CABG is debatable, and there are only limited data supporting this. In addition, intense pre-operative evaluation of patient risk factors, careful assessment of supra-aortic vessels and ascending aorta for atherosclerotic disease, use of off-pump “no-touch aorta” surgery, monitoring of cerebral oximetry for early detection and treatment of cerebral hypoxia, and prevention and treatment of post-operative atrial fibrillation may reduce the risk of perioperative stroke in CABG-treated patients [25, 26].
Recently, Jamie et al. investigated whether high-risk patients with LMCAD and prior CEVD preferentially benefit from revascularization by PCI compared with CABG in the EXCEL trial. They demonstrated that patients with LMCAD and prior CEVD, when compared with those without CEVD, had higher rates of stroke and reduced event-free survival after revascularization, irrespective of the mode of the revascularization. Overall, patients with prior CEVD had higher rates of stroke at 30 days (2.2 vs. 0.8%; p = 0.05) and 3 years (6.4 vs. 2.2%; p = 0.0003) and higher 3 year rates of the primary endpoint of all-cause death, stroke, or myocardial infarction (25.0 vs. 13.6%; p < 0.0001) [14]. Notably, no data pertaining to the impact of previous CEVD on very long-term (up to 10 years) mortality after revascularization in patients with 3VD and/or LMCAD are available. Not surprisingly, in our present analyses, we demonstrated that prior CEVD was associated with a significantly increased risk of all-cause death at 10 years, with no significant interaction between prior CEVD and revascularization strategy for the relative risk of all-cause death at 10 years. These findings do not support the strategy that patients with prior CEVD should be preferentially referred for PCI rather than CABG. Instead, the heart team [27] should assess the risk/benefit ratio of CABG versus PCI, by considering the periprocedural surgical risk, anatomical complexity, possibility for complete revascularization, potential procedural complications, benefits of each treatment strategy that emerge over time (beyond the periprocedural period), and patient preferences[28] when selecting the optimal revascularization strategy for 3VD and/or LMCVD patients with prior CEVD.
Limitations
Our findings should be interpreted in light of the following limitations. First, the present study is a post hoc analysis and should be considered as hypothesis-generating only [29]. In the multivariate analysis, a variety of available confounders have been adjusted for, even though, some may exist that may have not been identified. Second, the prior CEVD was site reported and the screening for CEVD was left to the discretion of each physician, which could lead to an underestimation of the rate of CEVD. Third, the number of patients with prior CEVD was relatively small (n = 253) and the present subgroup analysis may, thereby, be underpowered [29]. Therefore, further studies with large sample sizes are warranted to compare the relative treatment benefit of PCI or CABG at extended long-term follow-up. In addition, lacking follow-up stroke data and the functional neurological outcomes was another major limitation of the SYNTAXES study. In our current analysis, some less severe CEVD was not included. We only evaluated the impact of the major CEVD on long-term all-cause death, which was consistent with most previous studies, and the major CEVD may more clinical relevant with the long-term outcomes [14]. Finally, the SYNTAX trial was conducted between 2005 and 2007 with use of the first-generation drug eluting stents that were then available for treatment with PCI, which may limit generalizability of our findings to contemporary clinical practice [30]. Nevertheless, the SYNTAXES study, which achieved a relatively high follow-up rate (94%), is the first one to provide randomized data on the 10 year vital status of patients included in the trial [10].
Conclusions
Presence of prior CEVD in patients with 3VD and/or LMCVD planned for a revascularization procedure represents a high-risk patient group with complex and diffuse CAD and multiple comorbidities. A history of CEVD was associated with a significantly increased risk of all-cause death at 10 years following PCI or CABG. The risk of all-cause death at 10 years in patients having PCI or CABG was not significantly different according to CEVD status. The current findings from the SYNTAXES study do not support preferential referral for PCI rather than CABG in this population on the basis of a history of prior CEVD. Instead, decision making needs to include assessment of both short- and long-terms risks while discussing strategies amongst care providers and with patients.
Abbreviations
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CEVD:
-
Cerebrovascular disease
- LMCAD:
-
Left main coronary artery disease
- PCI:
-
Percutaneous coronary intervention
- RCTs:
-
Randomized controlled trials
- TIA:
-
Transient ischemic attack
- 3VD:
-
Three-vessel disease
References
Craven TE, Ryu JE, Espeland MA et al (1990) Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation 82(4):1230–1242. https://doi.org/10.1161/01.cir.82.4.1230
Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY (2013) Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis 227(2):193–200. https://doi.org/10.1016/j.atherosclerosis.2012.11.008
Morikami Y, Natsuaki M, Morimoto T et al (2013) Impact of polyvascular disease on clinical outcomes in patients undergoing coronary revascularization: an observation from the CREDO-Kyoto Registry Cohort-2. Atherosclerosis 228(2):426–431. https://doi.org/10.1016/j.atherosclerosis.2013.04.005
Eagle KARC, Foster ED, Mickel MC, Gersh BJ (1994) Long-term survival in patients with coronary artery disease: importance of peripheral vascular disease. The coronary artery surgery study (CASS) investigators. J Am Coll Cardiol. 23:1091–1095
Nallamothu BK, Chetcuti S, Mukherjee D et al (2003) Long-term prognostic implication of extracardiac vascular disease in patients undergoing percutaneous coronary intervention. Am J Cardiol 92(8):964–966. https://doi.org/10.1016/s0002-9149(03)00978-0
Palmerini T, Biondi-Zoccai G, Reggiani LB et al (2012) Risk of stroke with coronary artery bypass graft surgery compared with percutaneous coronary intervention. J Am Coll Cardiol 60(9):798–805. https://doi.org/10.1016/j.jacc.2011.10.912
Head SJ, Milojevic M, Daemen J et al (2018) Stroke rates following surgical versus percutaneous coronary revascularization. J Am Coll Cardiol 72(4):386–398. https://doi.org/10.1016/j.jacc.2018.04.071
Palmerini T, Biondi-Zoccai G, Riva DD et al (2013) Risk of stroke with percutaneous coronary intervention compared with on-pump and off-pump coronary artery bypass graft surgery: Evidence from a comprehensive network meta-analysis. Am Heart J. 165(6):910–7.e14. https://doi.org/10.1016/j.ahj.2013.03.011
Kang SH, Lee CW, Lee JB et al (2017) Mortality of patients with previous stroke undergoing drug-eluting stent implantation. Coron Artery Dis 28(7):543–549. https://doi.org/10.1097/MCA.0000000000000528
Thuijs D, Kappetein AP, Serruys PW et al (2019) Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet. https://doi.org/10.1016/S0140-6736(19)31997-X
Ong AT, Serruys PW, Mohr FW et al (2006) The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: design, rationale, and run-in phase. Am Heart J 151(6):1194–1204. https://doi.org/10.1016/j.ahj.2005.07.017
Serruys PW, Morice MC, Kappetein AP et al (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360(10):961–972. https://doi.org/10.1056/NEJMoa0804626
Mohr FW, Morice MC, Kappetein AP et al (2013) Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 381(9867):629–638. https://doi.org/10.1016/S0140-6736(13)60141-5
Diamond J, Madhavan MV, Sabik JF 3rd et al (2018) Left main percutaneous coronary intervention versus coronary artery bypass grafting in patients with prior cerebrovascular disease: results from the EXCEL trial. JACC Cardiovasc Interv 11(24):2441–2450. https://doi.org/10.1016/j.jcin.2018.09.008
Hillen T, Coshall C, Tilling K et al (2003) Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke 34(6):1457–1463. https://doi.org/10.1161/01.STR.0000072985.24967.7F
Song C, Sukul D, Seth M et al (2018) Outcomes after percutaneous coronary intervention in patients with a history of cerebrovascular disease: insights from the blue cross blue shield of michigan cardiovascular consortium. Circ Cardiovasc Interv 11(6):e006400. https://doi.org/10.1161/CIRCINTERVENTIONS.118.006400
Tarakji KGSIJ, Bhudia SK, Batizy LH, Blackstone EH (2011) Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA 305:381–390
Mack MJ, Head SJ, Holmes DR Jr et al (2013) Analysis of stroke occurring in the SYNTAX trial comparing coronary artery bypass surgery and percutaneous coronary intervention in the treatment of complex coronary artery disease. JACC Cardiovasc Interv 6(4):344–354. https://doi.org/10.1016/j.jcin.2012.11.010
Head SJ, Davierwala PM, Serruys PW et al (2014) Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J. 35(40):2821–2830. https://doi.org/10.1093/eurheartj/ehu213
Lee CW, Ahn JM, Cavalcante R et al (2016) Coronary artery bypass surgery versus drug-eluting stent implantation for left main or multivessel coronary artery disease: a meta-analysis of individual patient data. JACC Cardiovasc Interv 9(24):2481–2489. https://doi.org/10.1016/j.jcin.2016.10.008
Hata R, Kubo S, Tsuneyoshi H et al (2019) Long-term outcomes of three-vessel coronary artery disease after coronary revascularization by percutaneous coronary intervention using second-generation drug-eluting stents versus coronary artery bypass graft surgery. Cardiovasc Interv Ther. https://doi.org/10.1007/s12928-019-00599-5
Sasao H, Fujiwara H, Horiuchi N et al (2015) Comparison of long-term clinical outcomes after drug-eluting stent implantation in patients with coronary artery disease with and without prior cerebral infarction. Ann Vasc Dis 8(2):79–86. https://doi.org/10.3400/avd.oa.14-00137
Piccolo R, Giustino G, Mehran R, Windecker S (2015) Stable coronary artery disease: revascularisation and invasive strategies. The Lancet 386(9994):702–713. https://doi.org/10.1016/s0140-6736(15)61220-x
Doenst T, Haverich A, Serruys P et al (2019) PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol 73(8):964–976. https://doi.org/10.1016/j.jacc.2018.11.053
Wahba A, Milojevic M, Boer C et al (2020) 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg 57(2):210–251. https://doi.org/10.1093/ejcts/ezz267
Gaudino M, Angiolillo DJ, Di Franco A et al (2019) Stroke after coronary artery bypass grafting and percutaneous coronary intervention: incidence, pathogenesis, and outcomes. J Am Heart Assoc 8(13):e013032. https://doi.org/10.1161/JAHA.119.013032
Windecker S, Neumann FJ, Juni P, Sousa-Uva M, Falk V (2019) Considerations for the choice between coronary artery bypass grafting and percutaneous coronary intervention as revascularization strategies in major categories of patients with stable multivessel coronary artery disease: an accompanying article of the task force of the 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 40(2):204–212. https://doi.org/10.1093/eurheartj/ehy532
Kipp R, Lehman J, Israel J, Edwards N, Becker T, Raval AN (2013) Patient preferences for coronary artery bypass graft surgery or percutaneous intervention in multivessel coronary artery disease. Catheter Cardiovasc Interv 82(2):212–218. https://doi.org/10.1002/ccd.24399
Milojevic M, Nikolic A, Juni P, Head SJ (2020) A statistical primer on subgroup analyses. Interact Cardiovasc Thorac Surg. https://doi.org/10.1093/icvts/ivaa042
Escaned J, Collet C, Ryan N et al (2017) Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J 38(42):3124–3134. https://doi.org/10.1093/eurheartj/ehx512
Acknowledgements
None.
Funding
The SYNTAX Extended Survival study was supported by the German Foundation of Heart Research (Frankfurt am Main, Germany). The SYNTAX trial, during 0–5 year follow-up, was funded by Boston Scientific Corporation (Marlborough, MA, USA). Both sponsors had no role in the study design, data collection, data analyses and interpretation of the study data, nor were involved in the decision to publish the final manuscript. The principal investigators and authors had complete scientific freedom.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Serruys reports personal fees from Biosensors, Micel Technologies, Sinomedical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. Dr. Kappetein reports to work as an employee of Medtronic, outside the submitted work. Dr. Head reports to work as a full-time employee of Medtronic outside the scope of this work. Dr. Morice reports to work as the CEO of CERC, a CRO which was never involved in the SYNTAX trial at any level, except that submitted the 10 years additional follow-up (for free) to French authorities to get approval. Dr. van Geuns reports personal fees from Abbott vascular, grants and personal fees from AstraZeneca, grants and personal fees from Amgen, grants and personal fees from Boston Scientific, personal fees from Sanofi, outside the submitted work. All other authors have no disclosures.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Takahashi, K., Garg, S. et al. Ten-year all-cause death following percutaneous or surgical revascularization in patients with prior cerebrovascular disease: insights from the SYNTAX Extended Survival study. Clin Res Cardiol 110, 1543–1553 (2021). https://doi.org/10.1007/s00392-020-01802-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-020-01802-x