Abstract
Background: Major bleeding (MB) events are independent predictors of mortality after cardiac interventional procedures. The clinical relevance of MB following left atrial appendage occlusion (LAAO) remains unclear.
Aims: This study aimed to investigate the incidence and clinical impact of MB after LAAO in a real-world population at high risk for bleeding and contraindicated to anticoagulation.
Methods: The two-year results of the Amplatzer Amulet Observational Post-Market Study were analysed. An independent committee adjudicated MBs according to the Bleeding Academic Research Consortium scale. Cox proportional hazards regression identified variables associated with MB events and mortality.
Results: The MB rate was 7.2%/year, with a rate of 10.1%/year during year one, decreasing to 4.0%/year over year two. The most common bleeding location was gastrointestinal, accounting for 48% of MBs. Pre-LAAO MB was associated with an increased risk for post-LAAO MB (HR 2.34, 95% CI: 1.37-3.99). The occurrence of post-LAAO MB was associated with increased mortality (37.3% vs 12.7%; p<0.0001), driven mainly by events occurring beyond the periprocedural period. The annualised rate of ischaemic stroke or TIA was similar in patients with and without MB (2.3% vs 3.3%; p=0.446). MB post LAAO was a strong independent predictor of mortality (HR 3.07, 95% CI: 2.15-4.40).
Conclusions: In real-world patients at high bleeding risk, MB following LAAO was not uncommon and associated with a significant increase in mortality, without increasing the risk of stroke. ClinicalTrials.gov Identifier: NCT02447081. https://clinicaltrials.gov/ct2/show/NCT02447081
Introduction
Percutaneous left atrial appendage occlusion (LAAO) is increasingly applied for stroke prevention in patients with non-valvular atrial fibrillation (AF) and having a relative or absolute contraindication to long-term oral anticoagulation (OAC)1,2,3. Following LAAO, post-procedural antithrombotic therapy is recommended to prevent the occurrence of device-related thrombus, but it is usually restricted to a short period of time, potentially reducing the long-term risk of bleeding events. However, as LAAO is most often performed in patients with advanced age and multiple comorbidities, procedural and late bleeding events remain as potentially significant complications and could represent an area of improved patient care3,4. Despite this, little is known about the incidence, predictors and clinical impact of major bleeding (MB) after LAAO in a real-world setting of patients at high bleeding risk. Therefore, the aim of this study was to characterise the incidence, predictors and clinical impact of MB after LAAO in the prospective multicentre global Amplatzer™ Amulet™ Observational Post-Market Study.
Methods
This analysis utilised the complete two-year follow-up data of the prospective global Amplatzer Amulet Observational Post-Market Study3.
Study follow-up visits occurred at discharge, 1-3 months, 6 months, 1 year, and 2 years post procedure. At each visit, medications were reviewed, and it was ascertained if reportable adverse events had occurred. The protocol recommended subjects take aspirin for ≥6 months and clopidogrel per site standard of care following implant.
Serious adverse event reporting followed the International Organization for Standardization (ISO) 141555 definition. The sponsor provided source documentation concerning all serious adverse events to an independent clinical events committee (CEC) for adjudication. MB events were defined as type ≥3 on the Bleeding Academic Research Consortium (BARC) scale and categorised as major (3a, 3b, or 3c) or fatal (5a or 5b)5. The CEC adjudicated deaths as resulting from cardiovascular (CV), non-CV, or unknown causes. Deaths due to unknown causes are conservatively categorised as CV deaths in this analysis as per the Munich consensus document6.
STATISTICAL ANALYSIS
Descriptive statistics summarised baseline demographic and clinical characteristics. The Wilcoxon rank-sum test was used to identify differences in continuous variables between patients with and without MB events. Fisher’s exact test was used to identify differences in categorical variables. Annualised rates were defined as number of events per patient-years of follow-up and compared between groups using a Poisson regression model. The rates of MB, mortality, and the composite of ischaemic stroke or transient ischaemic attack (TIA) were calculated at two years post LAAO using the Kaplan-Meier method and compared between groups using the log-rank test. Cox proportional hazards regression was used to identify variables associated with MB events and mortality, reporting hazard ratios (HR) and 95% confidence intervals (CI). Variables with univariate p-values <0.20 were subsequently included in the multiple regression analyses. SAS 9.4 (SAS Institute, Cary, NC, USA) was used for analysis and Stata/SE 16.0 (StataCorp, College Station, TX, USA) for graphing. The lead author had the opportunity to query any aspect of the data and takes responsibility for data integrity and analysis.
Results
BASELINE CHARACTERISTICS
Most patients enrolled in the Amplatzer Amulet Observational Post-Market Study had a history of MB prior to LAAO implant (780/1,088 patients; 72%). The anatomical location of pre-LAAO MB events is detailed in Figure 1. Baseline characteristics for patients with and without MB over follow-up are presented in Table 1.
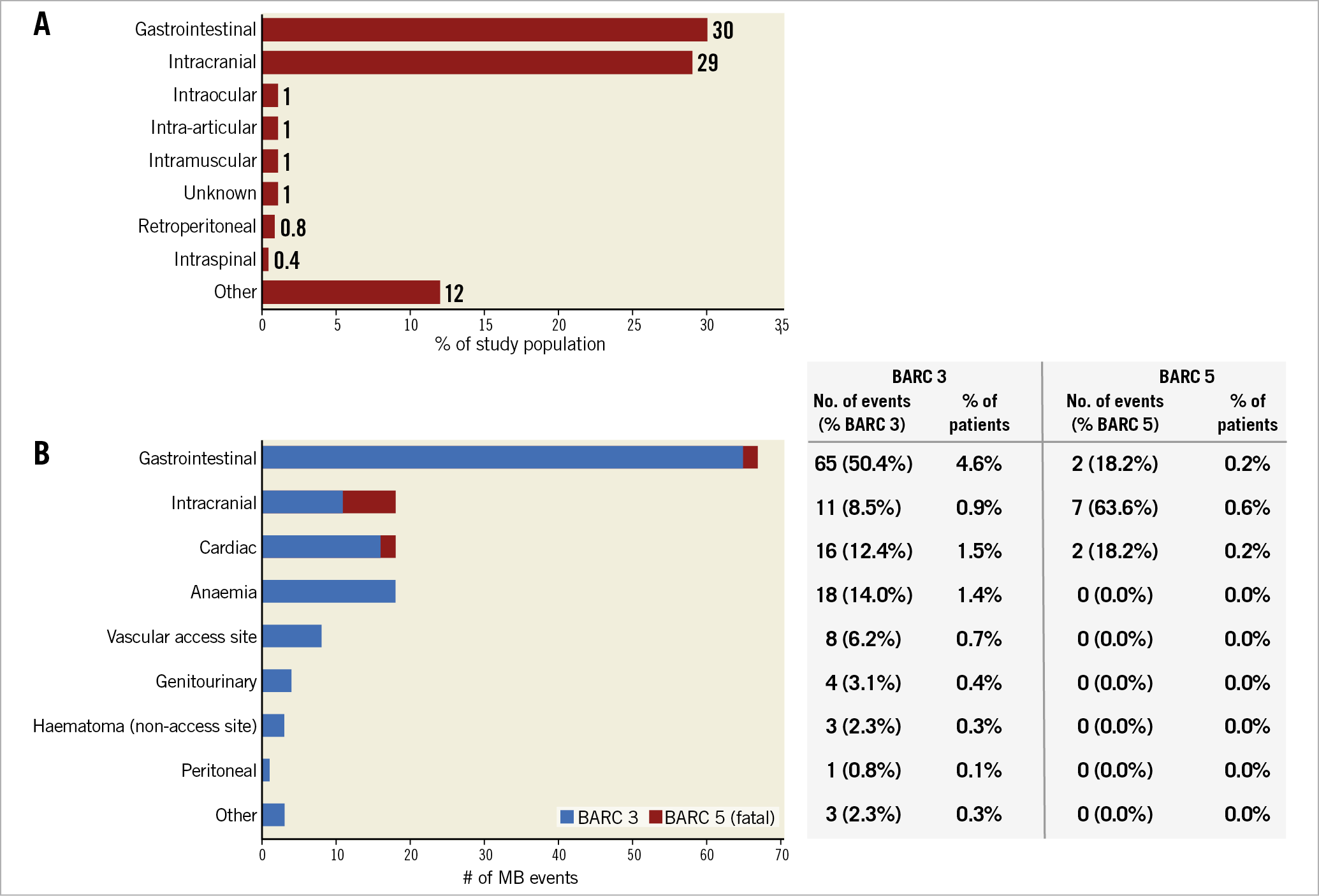
Figure 1. MB location and severity. A) The majority of pre-LAAO MBs were gastrointestinal or intracranial. B) Post LAAO, 67 of the 140 MBs were gastrointestinal, accounting for half of BARC 3 events and 18.2% of fatal BARC 5 events. Intracranial MBs occurred 18 times, with fatal BARC 5 events most often resulting from intracranial bleeds.
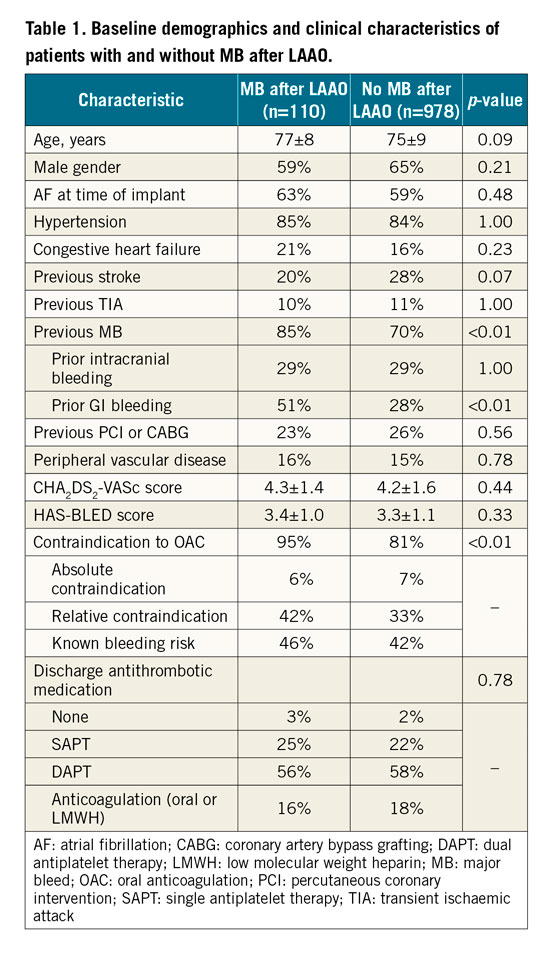
A previous history of MB, driven by prior gastrointestinal (GI) bleeding (51% vs 28%), and OAC contraindications (95% vs 81%) were more often present in patients experiencing MB during follow-up. The HAS-BLED (3.4±1.0 vs 3.3±1.1) and CHA2DS2-VASc scores (4.3±1.4 vs 4.2±1.6) were similar between the two groups.
ANTITHROMBOTIC MEDICATIONS
Most patients were discharged on dual (57.7%) or single (22.4%) antiplatelet therapy (APT), while 17.8% were discharged on anticoagulants3. Of note, no difference was found in the antithrombotic medication regimen at discharge from the index LAAO procedure in patients with and without MB after LAAO. At 45 days, 80.0% of those discharged on dual APT or anticoagulants still received the intensive therapy. At six months, most subjects transitioned to single APT (61.8%) or no antithrombotic medications (14.6%), with this regimen growing slightly over long-term follow-up up to two years (62.8%/21.5%, respectively).
Of patients with a known medication regimen at the time of pre-LAAO MB (745 of 780 patients), the majority (79.5%) were on an anticoagulant at the time of MB, while the remainder were on antiplatelet agents alone (9.1%) or no antithrombotic medications (11.4%). At the time of post-LAAO MB, 32.9% of patients were on dual APT, 31.4% on aspirin alone, 15.7% received no antithrombotic medications, 14.3% were on an anticoagulant, and 5.7% were on clopidogrel alone.
INCIDENCE, TIMING, AND RELATIONSHIP OF MB EVENTS
The CEC adjudicated 140 MB events in 110 subjects, which corresponds to an annualised rate of 7.2% and 10.1%, respectively, of patients undergoing an implant attempt. There were 30 patients who had an MB within 7 days of the procedure. Over half of the MB events (n=77) occurred within the first 90 days and 74% during the first year. The annualised MB rate decreased from 10.1% over the first year to 4.0%/year over the second year of follow-up. Figure 2 shows the cumulative rate of MB over time, as well as landmark analyses of bleeding rates over the periprocedural period (≤7 days), the intense antithrombotic period (6 months), and long-term follow-up (>6 months) when most subjects were maintained on a restricted antithrombotic regimen.
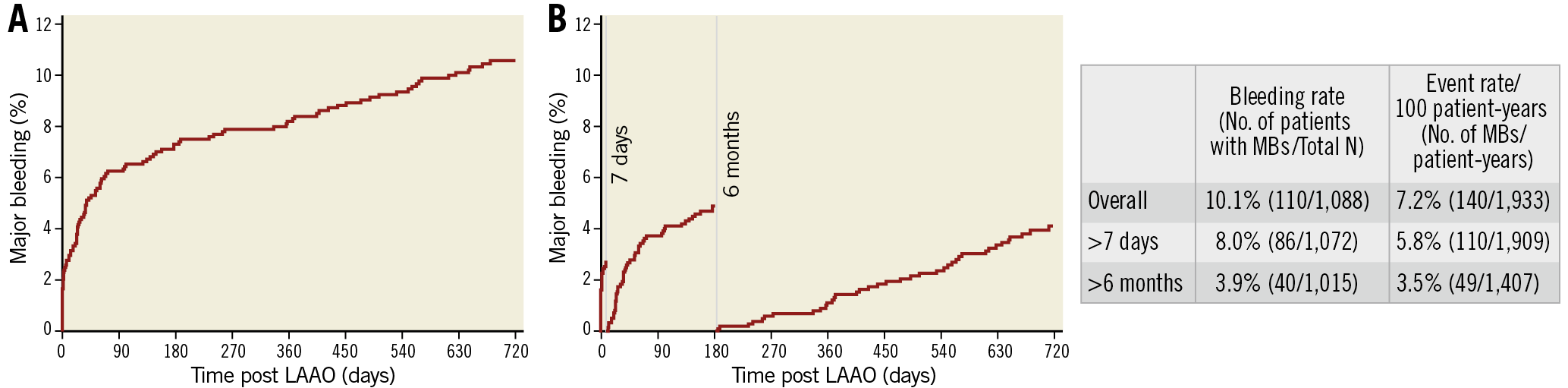
Figure 2. Cumulative and landmark analyses of major bleeding events after LAAO. A) During two years of follow-up, a total of 140 MB events occurred in 110 subjects, corresponding to an annualised rate of 7.2% and 10.1% of all patients, respectively. B) Three intervals: from LAAO procedure to day 7 (periprocedural), from day 8 to 6 months, during which most patients were still under an antithrombotic regimen, and long-term follow-up (>6 months) when most patients were maintained on a restricted antithrombotic regimen (single antiplatelet or no antithrombotic medication).
The CEC adjudicated 32 MB events (23%) as related to the AMPLATZER™ Amulet™ device (Abbott Vascular, Santa Clara, CA, USA) or the procedure, corresponding to 2.9% of enrolled patients. Of these, 27 MB events occurred ≤7 days from the index procedure. Pericardial effusion, pericardial tamponade, or cardiac perforations accounted for 15 of the device- or procedure-related MB events.
ANATOMICAL LOCATION AND SEVERITY OF MB EVENTS
Figure 1B shows the anatomical location of post-LAAO MB events, categorised by BARC severity. GI bleedings were the most common, accounting for 48% of all MB events. An active source of GI bleeding amenable to endoscopic treatment was only identified in 39% of cases. Intracranial (IC) bleeding, including intracerebral haemorrhage, subdural haematoma, and ischaemic stroke or brain tumour with haemorrhagic transformations, accounted for 13% of all MB events. Cerebral amyloid angiopathy was identified in one patient suffering an IC bleeding. Pericardial effusion, pericardial tamponade, or cardiac perforation occurred 16 times, consisting of 15 device- or procedure-related events. In addition, two aortic dissections brought the total number of cardiac bleeding events to 18.
Of 11 fatal bleeding events (BARC 5), 7 were due to IC bleeding, 2 were due to GI bleeding and 2 were due to cardiac-related events. Of note, only one fatal bleeding occurred within 7 days of LAAO - the result of intraprocedural cardiac perforation.
MORTALITY AND THROMBOEMBOLIC EVENTS IN PATIENTS WITH AND WITHOUT POST-LAAO MB
Kaplan-Meier analyses up to two years post LAAO compared mortality and thromboembolic events (ischaemic stroke or TIA) between patients with and without post-LAAO MB (Figure 3A-Figure 3C). Both all-cause (37.3% vs 12.7%; p<0.0001) and CV (25.2% vs 7.2%; p<0.0001) mortality rates at two years were significantly higher in patients with an MB event post LAAO.
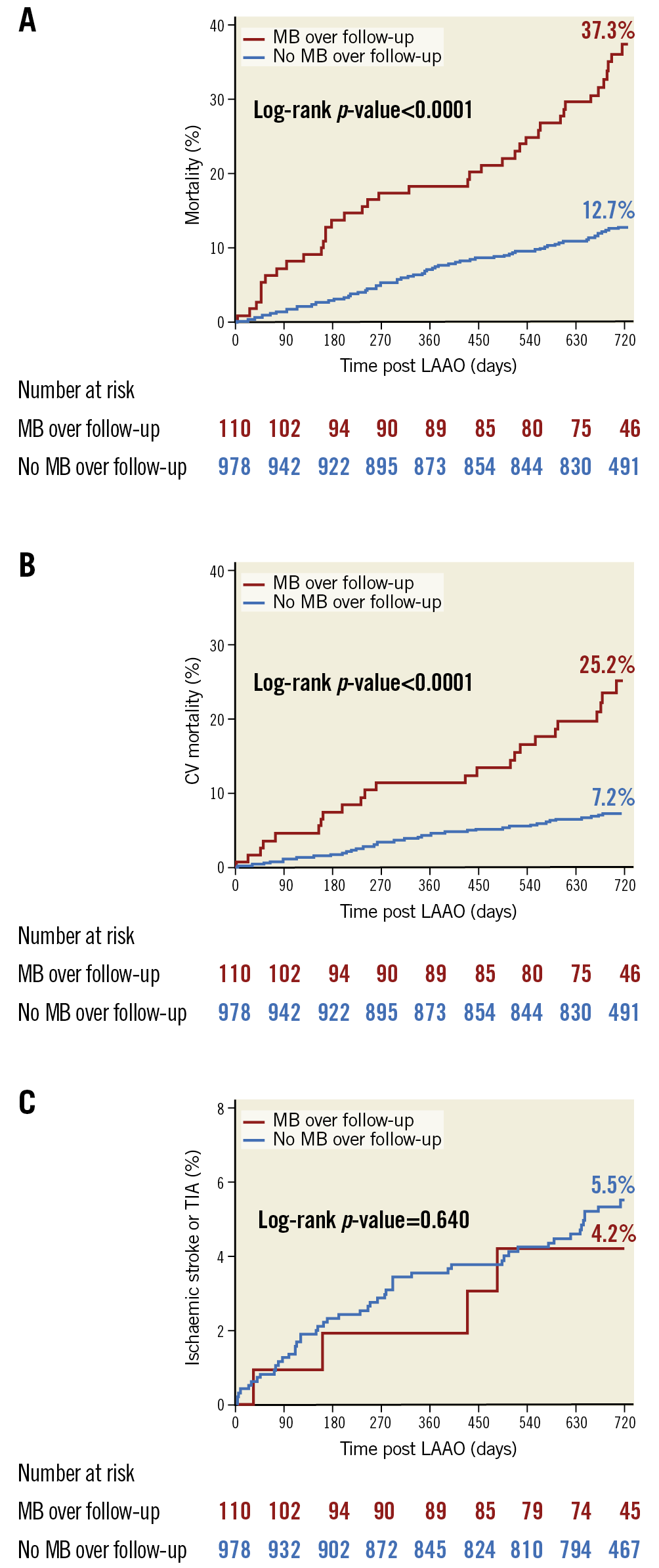
Figure 3. Association of post-LAAO major bleeding with clinical outcomes. Kaplan-Meier analyses up to two years post LAAO comparing all-cause mortality (A), CV mortality (B) and ischaemic stroke/TIA (C) between patients with and without MB events following LAAO. Both all-cause and CV mortality rates at two years were significantly higher in patients with an MB event post LAAO. The rate of ischaemic stroke or TIA was similar in patients with and without MB, with an annualised rate of ischaemic stroke of 1.7%/year and 2.2%/year, respectively (p=0.640).
The Kaplan-Meier rate of ischaemic stroke or TIA was similar in patients with and without MB (Figure 3C), with annualised rates of 2.3%/year and 3.3%/year (p=0.446), respectively.
Figure 4 shows mortality based upon the location of post-LAAO MB events. Patients with both a GI and other location MB event (n=7) or intracranial and other location MB event (n=1) over follow-up were counted in the GI and IC groups, respectively. At two years, the mortality rate was 64.7% for patients with IC bleeding, 34.1% for patients with GI bleeding and 29.3% for patients with an MB other than GI or IC.
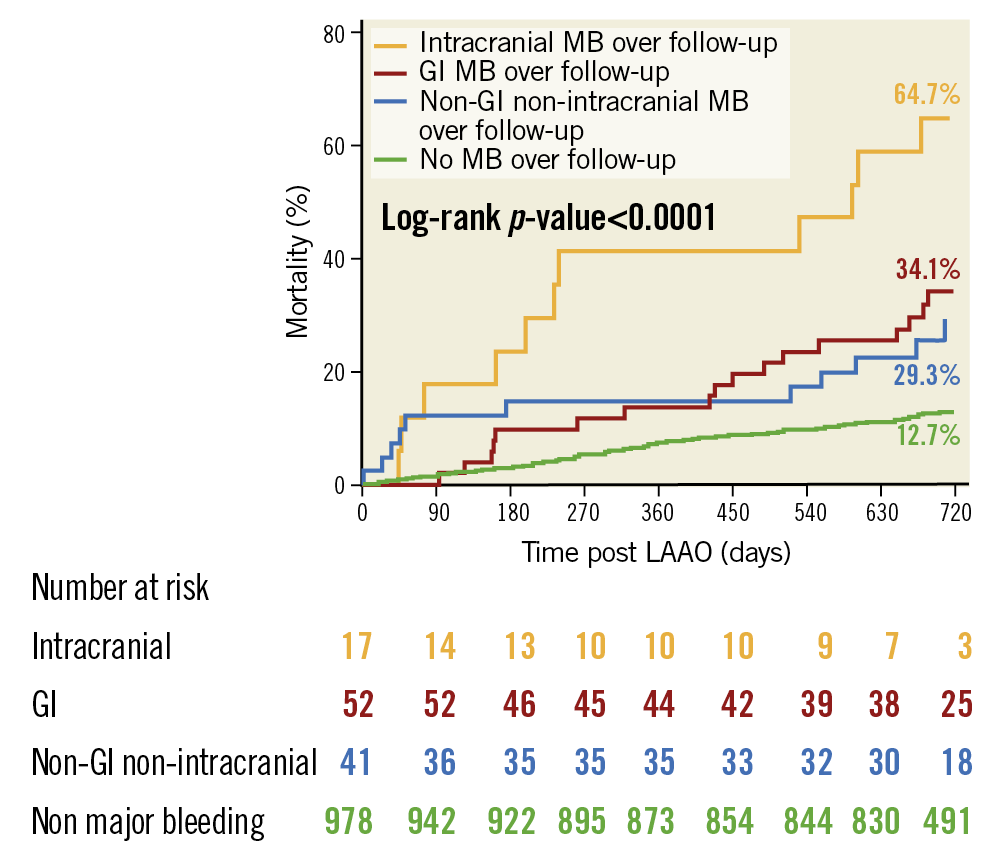
Figure 4. Association of major bleeding location with all-cause mortality. Patients were stratified according to MB location after LAAO: intracranial (IC), gastrointestinal (GI) and non-GI/non-IC. The cumulative mortality incidence for each subgroup is shown and compared to patients without MB.
Figure 5 illustrates the temporal relationship of MB events to death in the 41 patients who suffered an MB and died over follow-up. There were 11 patients with a BARC 5 bleeding event in whom the causal association between MB and death was ascertained by the CEC. In the remaining 30 patients with BARC 3 bleeding events, a time window of 30 days between MB and death was defined to imply a plausible causal relationship. A total of 8 additional patients had an MB event within this window. In the remaining 22 patients, there was no clear evidence of causality between MB and death. Of the 30 patients with a periprocedural (≤7 days) MB, only 1 died within 30 days post LAAO due to cardiac perforation (periprocedural MB-related mortality=0.1%; 1/1,088 patients).
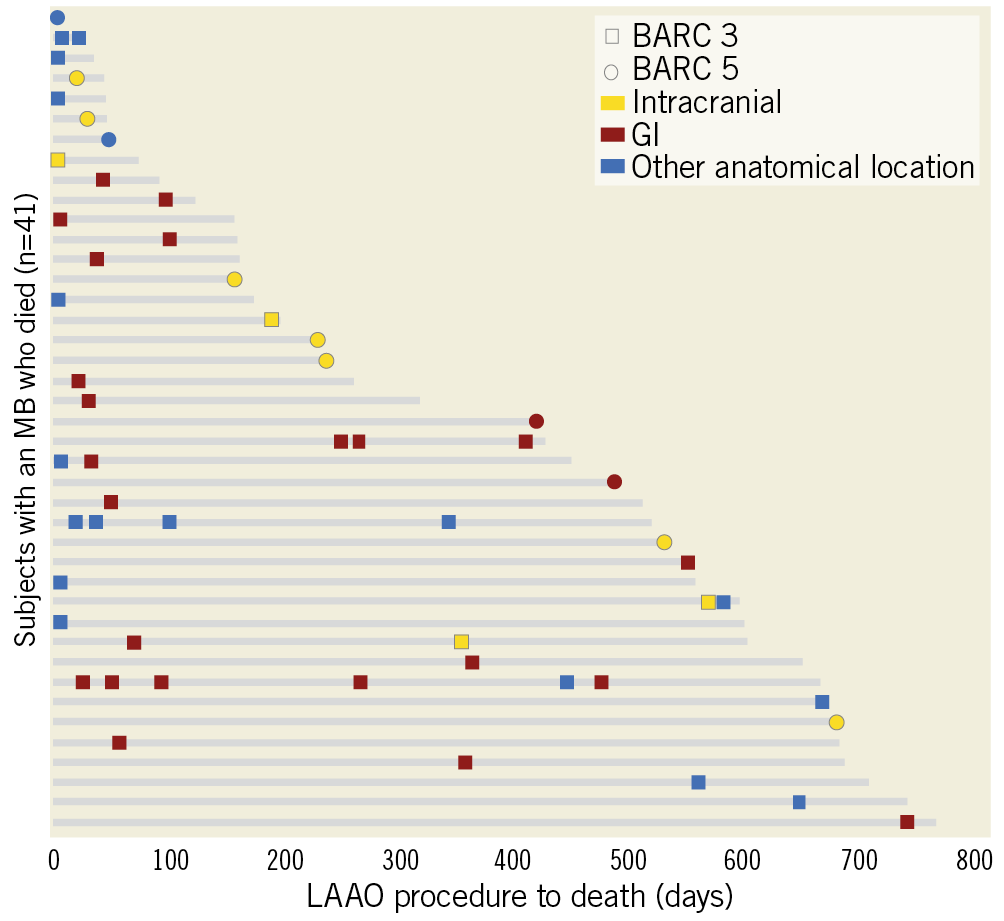
Figure 5. Major bleed timing, severity, and location relative to mortality. The 41 patients who had an MB and died over follow-up (grey lines) are shown. Periprocedural MBs were rarely fatal, with only one of the 30 patients with a periprocedural MB dying within 30 days of LAAO. Nineteen patients died within 30 days following an MB, with many of the late deaths occurring shortly after an IC or GI event.
PREDICTORS OF MB AND MORTALITY
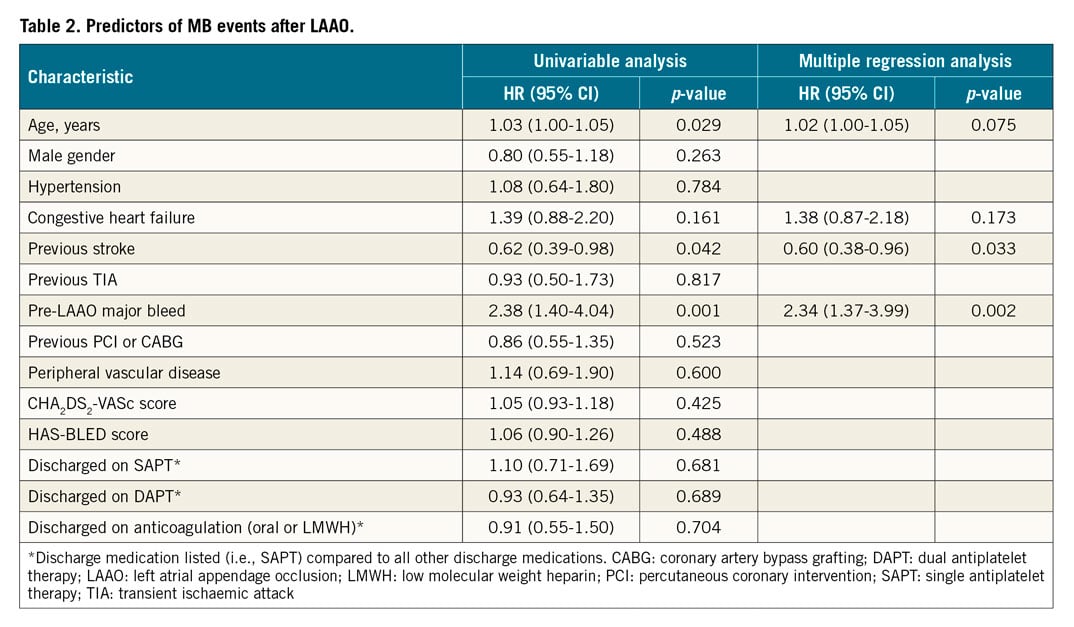
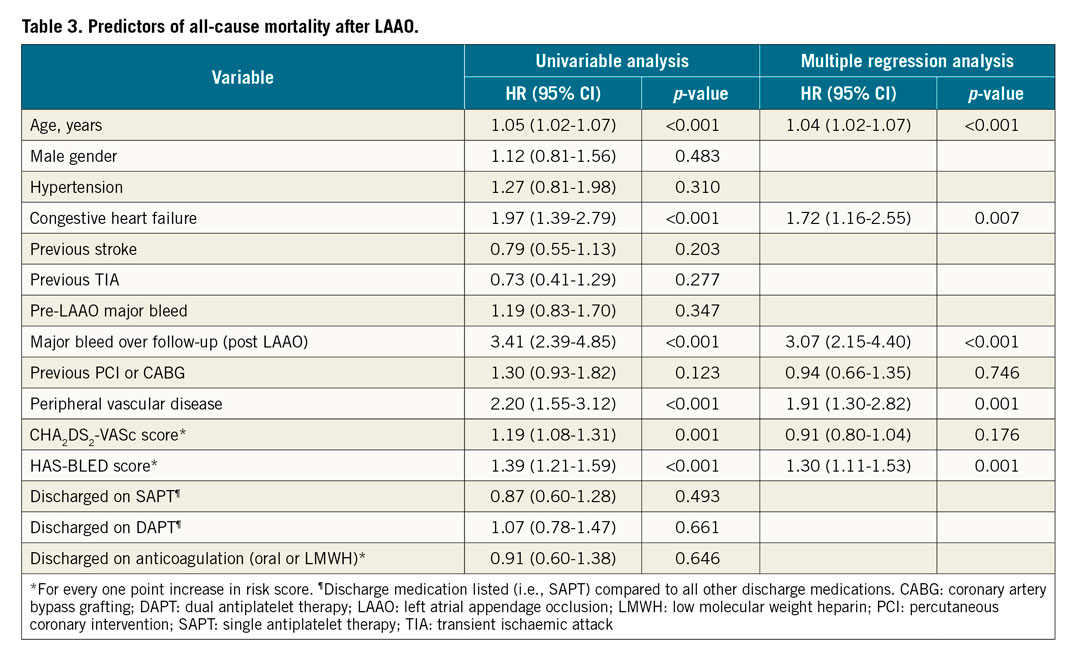
Univariable and multiple regression analyses were performed to identify predictors of MB events post LAAO (Table 2) and all-cause mortality (Table 3). Age, history of congestive heart failure, prior stroke, and prior MB were included in the multiple regression analysis to identify predictors of post-LAAO MB. A prior stroke reduced the risk for post-LAAO MB (HR 0.60, 95% CI: 0.38 to 0.96; p-value 0.033), while pre-LAAO MB increased the risk for post-LAAO MB (HR 2.34, 95% CI: 1.37 to 3.99; p-value 0.002). Multiple regression identified the following characteristics as predictors of all-cause mortality (Table 3) – age, congestive heart failure, MB post LAAO, peripheral vascular disease, and baseline HAS-BLED score.
Discussion
The main findings of the current study can be summarised as follows: 1) among a patient population at high bleeding risk treated with LAAO, MB events were not uncommon, with an annualised rate of 7.2%/year over a two-year follow-up; 2) patients with a prior history of MB were at higher risk of MB recurrence after the procedure; 3) the most common source of MB after LAAO was GI; 4) the occurrence of MB post LAAO was associated with a significant increase in the risk of all-cause and CV mortalities, driven mainly by bleeding events beyond the periprocedural period, without increasing the risk of stroke/TIA; and 5) MB was an independent predictor of all-cause mortality.
MB INCIDENCE AFTER LAAO AND COMPARISON WITH PUBLISHED STUDIES
In the current study, the annualised rate of MB was 10.1%/year during the first year, decreasing to 4.0%/year during the second year. The landmark analyses of Figure 2B show that, while the rate of MB decreases over time, there is still a continuous risk of MB despite a restrained antithrombotic regimen in this high bleeding risk population. Of note, 23% of all MB events were procedure- or device-related, occurring mostly during the periprocedural period. Pericardial effusion with or without tamponade and vascular complications accounted for the majority of procedure-/device-related MB events, emphasising the need for further improvements in procedural techniques and preventive strategies. Adequate physician training, better assessment of LAA anatomy by 3D imaging modalities, optimal device sizing, standardised implantation protocol and meticulous procedural anticoagulation all represent important aspects to mitigate not only acute procedure- or device-related MBs but also late pericardial effusions. In the AMPLATZER Cardiac Plug registry of 1,047 patients implanted with the first-generation AMPLATZER™ LAAO device (Abbott Vascular), the annualised rate of MB was lower at 2.1%/year1. However, cardiac tamponade occurred in 13 patients and was not counted as an MB event despite fulfilling the Munich consensus reporting criteria for an MB endpoint6. Moreover, the retrospective design of the study, without independent adjudication, may have resulted in significant under-reporting of bleeding events. In the two-year follow-up of the prospective EWOLUTION registry of 1,020 patients implanted with the WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA), the annualised rate of non-procedural MB was 2.7%/year, which did not include procedural bleeding events of tamponade or vascular complications2. Nevertheless, the higher than previously observed rate of MB in the current study could be related to the higher bleeding risk profile of the current study population as compared to previous studies. Indeed, a history of MB was present in 72% of the current study population, as compared to 47% in the ACP study and 31% in EWOLUTION. Interestingly, a specific analysis restricted to 318 patients with a prior MB in EWOLUTION showed higher rates of non-procedural MB at 4.5%/year, with a protracted risk reduction of 30% compared to the HAS-BLED score2. In a single-centre registry, Weise et al included 298 patients who underwent LAAO with five different LAAO devices (42% AMPLATZER)7. The mean HAS-BLED score was 3.5±1, with a high proportion of patients having a previous history of bleeding (61%), all reflecting patients with high bleeding risk profiles similar to our study population. Interestingly, they also reported a high incidence of MB events during follow-up at 10.7%. When cardiac tamponades were added, the incidence rose to as high as 14%. With regard to the anatomical location of MB in the current study, GI origin accounted for the vast majority of non-procedural MBs, similar to what has been reported after LAAO2,4,7 but also after percutaneous coronary intervention (PCI) and transcatheter aortic valve replacement (TAVR)8,9,10.
PREDICTORS OF MB AFTER LAAO
We found that prior stroke and pre-LAAO MB were independent predictors of MB during follow-up, while age had a borderline significance. Our findings suggest that patients with prior MB represent a population at increased risk of recurrent bleeding events after LAAO, requiring closer surveillance and improvement in bleeding avoidance strategies. However, by their nature, some bleeding conditions such as GI angiodysplasia and cerebral amyloid angiopathy will have a high probability of recurrence even after all antithrombotic treatments have been stopped11. Similar to EWOLUTION, the post-discharge antithrombotic treatment in the current study was not an independent predictor of MB, which could be related to the fact that the regimen was at physician discretion and patients with the highest risk of bleeding were probably discharged on single APT or even no treatment, whilst patients at lower risk were treated with dual APT or an anticoagulant. Of note, half of all MBs over the two years of follow-up occurred over the first 90 days, when 75.5% of patients were on dual APT or an anticoagulant. It is reasonable to assume that shorter durations of dual APT or anticoagulation after LAAO could decrease the long-term risk of bleeding events. In a propensity-adjusted analysis to assess the impact of dual APT duration on bleeding and thromboembolic events in the EWOLUTION trial, early discontinuation of dual APT (≤105 days) was associated with lower bleeding rates at 1.1%, while bleeding rates were higher at 3.5% for late discontinuation, with no difference in thromboembolic events2. Further studies are needed to assess the optimal duration and type of antithrombotic treatment after LAAO, balancing the risk of bleeding with that of thromboembolic events and device-related thrombus (DRT). In the current study, the rate of DRT at two years was 1.6%3, with only one patient having both a DRT and an MB. As previously detailed in a stand-alone DRT sub-ana-ly-sis12, the discharge antithrombotic regimen was similar between patients with and without DRT, APT agents alone were used when 77% of DRTs were identified, and 83% of patients received anticoagulation to resolve the DRT. Fortunately, no MBs occurred in patients following DRT despite the use of anticoagulation in this high-risk group.
CLINICAL IMPACT OF MB AFTER LAAO
Periprocedural and late bleeding complications have been associated with early and late mortality following PCI and TAVR9,13. To the best of our knowledge, our study is the first specifically to evaluate the clinical impact of MB after LAAO. We found that MB following LAAO was associated with markedly increased risks for all-cause and CV mortality, without increasing the risk of stroke/TIA. Moreover, MB was found to be an independent predictor of all-cause mortality. A causal relationship between MB and death was confirmed in 11 patients and suspected in another 8 patients by using a time window of 30 days between MB and death. Although GI bleeding was the most common location of MB, and associated with a high mortality rate, the fatality rate was highest for IC bleeding, with most deaths occurring shortly after IC bleeding. As LAAO is intended to be a lifelong preventive procedure against stroke, most of the attention has been focused on the improvement in procedural safety and acute outcomes. Accordingly, we found that bleeding events that occurred during the periprocedural period were less likely to result in fatal outcome, as most events occurred in the hospital setting where prompt care is readily available. The current study underscores the detrimental effect of MB beyond the periprocedural period and identifies an area of improved patient care. Prevention and reduction of MB could represent an important future target to improve the long-term prognosis after LAAO. Beyond MB, all other independent predictors of all-cause mortality (increasing age, congestive heart failure, PVD and HAS-BLED score) pointed to an increase in patient risk profile and comorbidities, all factors contributing to worse long-term prognosis.
Study strengths and limitations
This study has some important strengths. It is the largest prospective real-world LAAO study to use an independent CEC, providing a systematic, standardised and independent characterisation of bleeding events. Nevertheless, some limitations should be acknow-ledged. First, although antithrombotic therapy at discharge did not influence the risk of MB, our results must be interpreted in light of the non-randomised nature of the study. Therefore, patients at highest risk of bleeding may have received less potent antithrombotic therapy post LAAO and the opposite holds true for patients at lower bleeding risk. Additionally, although we have adjusted the risk of mortality for imbalances in a number of important covariates, potential unmeasured confounders may still be present.
Conclusions
In a real-world population of patients at high risk for bleeding who underwent LAAO, MB occurred in 10.1% and was associated with a significant increase in all-cause and CV mortality, without increasing the risk of stroke. Given the growing adoption of LAAO, preventive strategies, early detection and prompt management of MB could represent an important future target to improve long-term prognosis after LAAO.
|
Impact on daily practice In patients at high bleeding risk undergoing LAAO, post-procedural major bleeding was not uncommon and associated with a significant increase in all-cause mortality, driven mainly by events occurring beyond the periprocedural period. Strategies to limit bleeding events, potentially including a restrained adjunctive antithrombotic regimen, early detection and prompt management of events could all represent important targets to reduce the downstream impact of major bleeding following LAAO. |
Acknowledgements
The authors wish to thank Hong Zhao, PhD, and Jingyun Li, MS, from Abbott for statistical analysis.
Funding
Abbott provided funding for the Amplatzer Amulet Observational Post-Market Study. No funding was provided for this substudy analysis.
Conflict of interest statement
A. Aminian has served as a proctor for Abbott and is a consultant for Abbott. O. De Backer has received institutional research grants and consultation fees from Abbott. J.E. Nielsen-Kudsk has served as a proctor for Abbott and is a consultant for Abbott and Boston Scientific. P. Mazzone has served as a consultant for Abbott, Boston Scientific, and Medtronic. S. Berti has served as a proctor for Abbott. S. Fischer has served as a proctor for Biotronik and Boston Scientific and is a consultant for Abbott. J. Lund has served as a proctor for Abbott. M. Montorfano has served as a proctor for Abbott, Boston Scientific, and Edwards Lifesciences. X. Freixa has served as a proctor and consultant for Abbott. R. Gage is an employee of Abbott. H.C. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, as co-editor of Cephalalgia and on the editorial board of Lancet Neurology, Stroke, European Neurology and Cerebrovascular Disorders. H.C. Diener chairs the Treatment Guidelines Committee of the German Society of Neurology and contributed to the EHRA and ESC guidelines for the treatment of AF. B. Schmidt has served as a consultant for Boston Scientific and Medtronic. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.




