
Abstract
Aims: Left atrial appendage closure (LAAC) with AMPLATZER occluders is used for stroke prevention in atrial fibrillation (AF). Net clinical benefit compared to medical therapy has not been tested. The aim of this study was to test whether long-term clinical outcome after LAAC with AMPLATZER occluders may be similar to medical therapy.
Methods and results: Five hundred consecutive patients who underwent LAAC with AMPLATZER occluders were compared to 500 patients with medical therapy by propensity score matching. The primary efficacy endpoint was a composite of stroke, systemic embolism and cardiovascular/unexplained death. The primary safety endpoint consisted of major procedural adverse events and major bleedings. For assessment of net clinical benefit, all of the above-mentioned hazards were combined. After 2,645 patient-years at a mean follow-up of 2.7±1.5 years, the primary efficacy endpoint was reached by 75/1,342, 5.6% in the LAAC group versus 102/1,303, 7.8% per 100 patient-years (hazard ratio [HR] 0.70, 95% confidence interval [CI]: 0.53-0.95, p=0.026). The primary safety endpoint occurred in 48/1,342, 3.6% versus 60/1,303, 4.6% per 100 patient-years (HR 0.80, 95% CI: 0.55-1.18, p=0.21), and the combined hazard endpoint in 109/1,342, 8.1% versus 142/1,303, 10.9% per 100 patient-years (HR 0.76, 95% CI: 0.60-0.97, p=0.018). Patients receiving LAAC demonstrated lower rates of both all-cause and cardiovascular mortality (111/1,342, 8.3% vs 151/1,303, 11.6% per 100 patient-years [HR 0.72, 95% CI: 0.56-0.92, p=0.005] and 54/1,342, 4.0% vs 84/1,303, 6.5% per 100 patient-years [HR 0.64, 95% CI: 0.46-0.89, p=0.007]).
Conclusions: LAAC with AMPLATZER devices showed a net clinical benefit over medical therapy by superior efficacy, similar safety and a benefit in all-cause and cardiovascular mortality.
Introduction
Non-valvular atrial fibrillation (AF) is the most common arrhythmia with a prevalence of 1%-2% in the general population, increasing with age and affecting approximately 7% of individuals aged >65 years and 15%-20% of octogenarians1,2. In comparison to non-cardioembolic strokes, a larger amount of cerebral tissue is affected by the larger cardiac emboli, resulting in larger areas of ischaemia with considerable disability and high mortality rates3,4,5. Therefore, medical therapy with oral anticoagulation (OAC) by vitamin K antagonists (VKA) or non-vitamin K antagonists (NOAC) is the mainstay for cardioembolic stroke prevention. The left atrial appendage (LAA) is the main source of thrombus formation in patients with AF who have suffered a stroke. Given the limitations of antithrombotic medical therapy, percutaneous left atrial appendage closure (LAAC) has evolved as an alternative option6. Based on superior efficacy, non-inferior safety and superior all-cause and cardiovascular mortality in comparison to warfarin from two randomised trials, the WATCHMAN™ occluder (Boston Scientific, Marlborough, MA, USA) was approved by the US Food and Drug Administration in March 2015 for patients with AF who are eligible for OAC7,8,9. Besides the WATCHMAN, the AMPLATZER™ occluders (Abbott Vascular, Santa Clara, CA, USA) are widely used for LAAC, typically in patients not amenable to OAC6,10,11,12. So far, only registry data of LAAC with AMPLATZER systems with a long-term follow-up have been published13; there have been no randomised trials. Therefore, the objective of the present propensity score-matched study was to test whether long-term clinical outcome after LAAC with AMPLATZER occluders may be similar to medical therapy.
Methods
STUDY DESIGN
APPLY (ClinicalTrials.gov: NCT02787525) was a dual-centre observational retrospective study with a logistic 1:1 nearest neighbour propensity score matching (PSM). It was conducted between 2016 and 2018 in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and the Swiss regulations on clinical research. The protocol was approved by the independent ethics committees at the two centres. All patients provided written informed consent prior to enrolment.
STUDY POPULATION
LAAC GROUP
The first 500 consecutive patients who underwent LAAC with dedicated AMPLATZER occluders between January 2009 and June 2015 at the Bern and Zurich university hospitals were entered into this prospective observational registry in line with current recommendations2,6,14,15. Exclusion criteria were overt infection, endocarditis, pregnancy, intracardiac thrombus, and reasons for OAC other than AF.
CONTROL GROUP
During the same time frame, 500 patients with AF and need for OAC served as the control group. These patients were recruited from the cardiology department at Bern University Hospital, where they were hospitalised during the years 2009 to 2015. Patients with known malignant conditions were excluded.
TREATMENT
LAAC GROUP
Device characteristics and procedural aspects have been described in detail previously10,14,16,17. LAAC was performed exclusively with AMPLATZER occluders: 403 patients (81%) received the first-generation AMPLATZER™ Cardiac Plug (ACP) and 97 (19%) the second-generation AMPLATZER™ Amulet™ (both Abbott Vascular). The left atrium was accessed by transseptal puncture in most patients or through a patent foramen ovale (PFO) or atrial septal defect (ASD) if present18. Antiplatelet treatment following LAAC consisted of dual antiplatelet therapy for one to six months, and thereafter single or no antiplatelet therapy, tailored to each patient’s bleeding risk.
CONTROL GROUP
Antithrombotic treatment usually consisted of OAC with VKA or NOAC. Since a substantial proportion of patients suffered from coronary artery disease in both groups, platelet inhibitors were frequently given continuously, in addition to OAC, in the medical group.
STUDY ENDPOINTS
In the LAAC group, demographic, clinical and procedural characteristics were prospectively collected in a dedicated database according to the current recommendations of the European Association of Percutaneous Cardiovascular Interventions6,14 and the Munich Consensus Document (MCD) on definitions, endpoints and data collection requirements14,15. The MCD criteria were established on the basis of the Valve Academic Research Consortium-2 (VARC-2) criteria and the Bleeding Academic Research Consortium (BARC) criteria19,20. For the control group, the same characteristics were captured retrospectively. All study endpoints were predefined and adopted from the PROTECT-AF trial7.
The primary safety endpoint was a composite of LAAC-related death, ischaemic stroke, cardiac tamponade, major access vessel complication, major device embolisation, severe kidney injury, need for cardiopulmonary resuscitation, need for urgent surgery (e.g., due to embolisation of the device, repair of procedure-related injury, or due to bleeding) and major or life-threatening bleeding according to VARC and BARC type 3a, 3b, 3c, or 5. The primary efficacy endpoint was a composite of stroke (non-disabling, disabling, ischaemic, haemorrhagic), systemic embolism, and cardiovascular or unexplained death.
For comparison of the net clinical benefit, the combined hazard endpoint was used. It is a composite of all hazards arising from the need for stroke protection by LAAC or anticoagulation, i.e., the above-mentioned LAAC-related complications, cardiovascular or unexplained death, any stroke, systemic embolism, pulmonary embolism, myocardial infarction and major or life-threatening bleeding. All event rates were calculated as the number of events per 100 patient-years of follow-up. Classification of death types was adjudicated according to the 2017 ACC/AHA 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials21.
Data collection, PSM and statistical analysis are provided in Supplementary Appendix 1.
Results
PATIENT POPULATION
Of the 1,000 AF patients enrolled in APPLY, 500 underwent LAAC with AMPLATZER devices, and 500 with standard medical therapy served as the matched control group (Table 1, Supplementary Table 1). PSM resulted in excellent bias reduction in all categories with absolute standardised differences <0.1 (Figure 1). Stroke risk and bleeding risk were high in both groups with HAS-BLED scores ≥3 in the majority of patients.
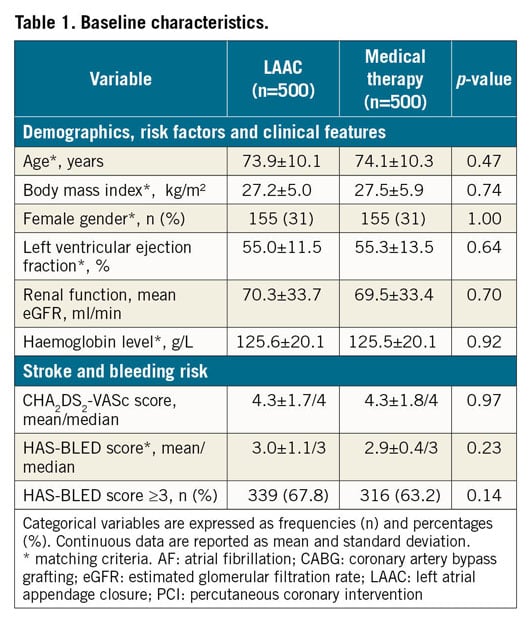
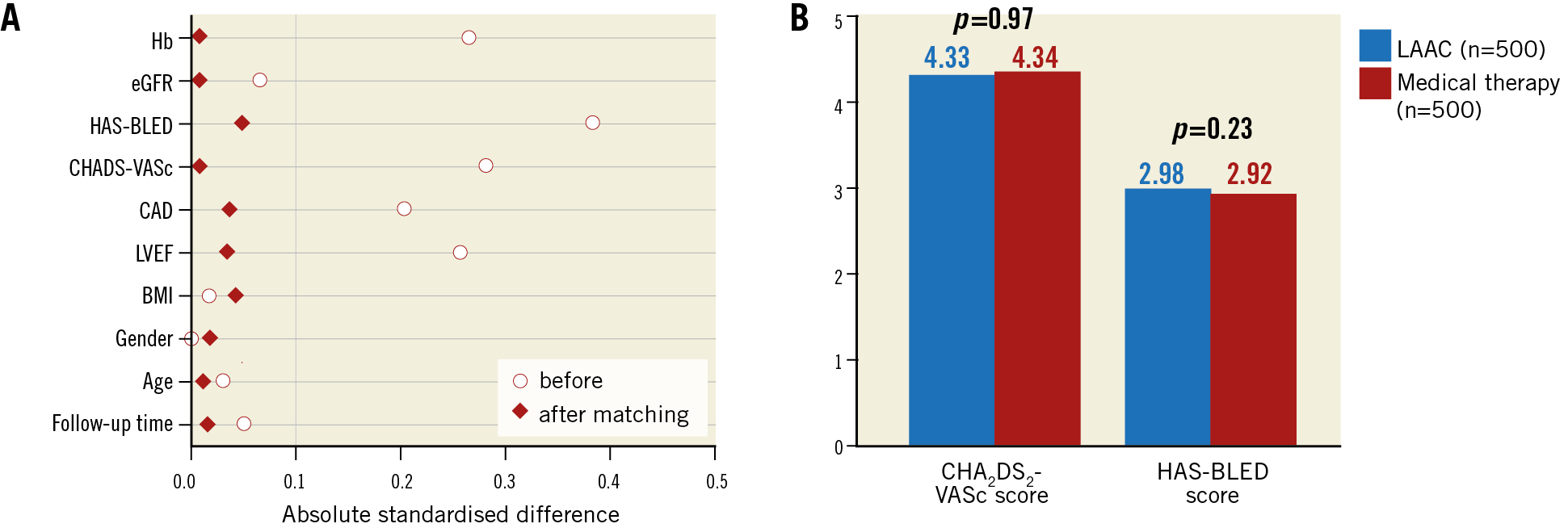
Figure 1. Results before and after matching. Good comparability by excellent bias reduction (A) in all categories with equalisation in baseline risk for stroke and bleeding (B).
In the LAAC group, device success was 98%, i.e., in 10 of 500 patients no occluder could be placed for different reasons. Since the present study follows the intention-to-treat principle, these remained in their respective group.
LONG-TERM CLINICAL OUTCOME
After a mean follow-up of 2.7±1.5 versus 2.6±1.5 years and a total of 2,645 patient-years (1,342 vs 1,303), clinical information was available for all 1,000 patients (Table 2, Supplementary Table 2, Supplementary Table 3). In the LAAC group, 7 of the 10 patients without an occluder were alive at follow-up: 4 of them underwent surgical LAAC, 1 was anticoagulated and 2 remained without any medical or device-based stroke protection. Thirty-nine of the 389 (10.0%) patients of the LAAC group received OAC apart from AF. In the control group, 275 of 349 (78.8%) were on VKA (53.6%) or NOAC (25.2%) (Supplementary Figure 1A). In 95 of 125 (76.0%) patients, international normalised ratio (INR) measurements were available; one or more measurements were out of therapeutic range (INR <2 or INR >3.5). Patients in the LAAC group had lower numbers of hospital stays (47.6% vs 69.8%, p<0.0001, mean number per patient 2.6±2.2 vs 3.0±2.4, p=0.016) and self-reported functional status was better in the LAAC group (Table 2, Supplementary Table 2, Supplementary Figure 1B).
PRIMARY EFFICACY ENDPOINT
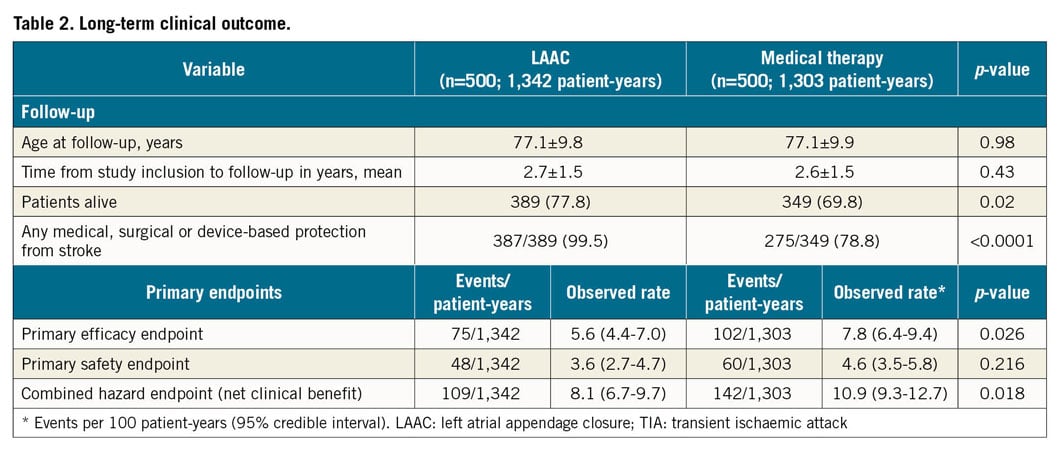
There were 75 primary efficacy events among the 500 LAAC patients during 1,342 patient-years, i.e., 5.6%, versus 102 events among the 500 patients of the control group during 1,303 patient-years, i.e., 7.8% per 100 patient-years (hazard ratio [HR] 0.70, 95% confidence interval [CI]: 0.53-0.95, p=0.026) (Figure 2A, Table 2). In the LAAC group, the incidence of stroke and transient ischaemic attack (TIA) tended to be lower (27/1,342, 2.0% vs 41/1,303, 3.2%, p=0.065) and, according to Cox regression analysis, the difference reached statistical significance (p=0.047) (Figure 2B, Figure 2C). Also, the rates of disabling and haemorrhagic strokes were lower in the LAAC group (9/1,342, 0.7% vs 20/1,303, 1.5%, p=0.033 and 1/1,342, 0.1% vs 6/1,303, 0.5%, p=0.053, respectively) (Figure 2D).
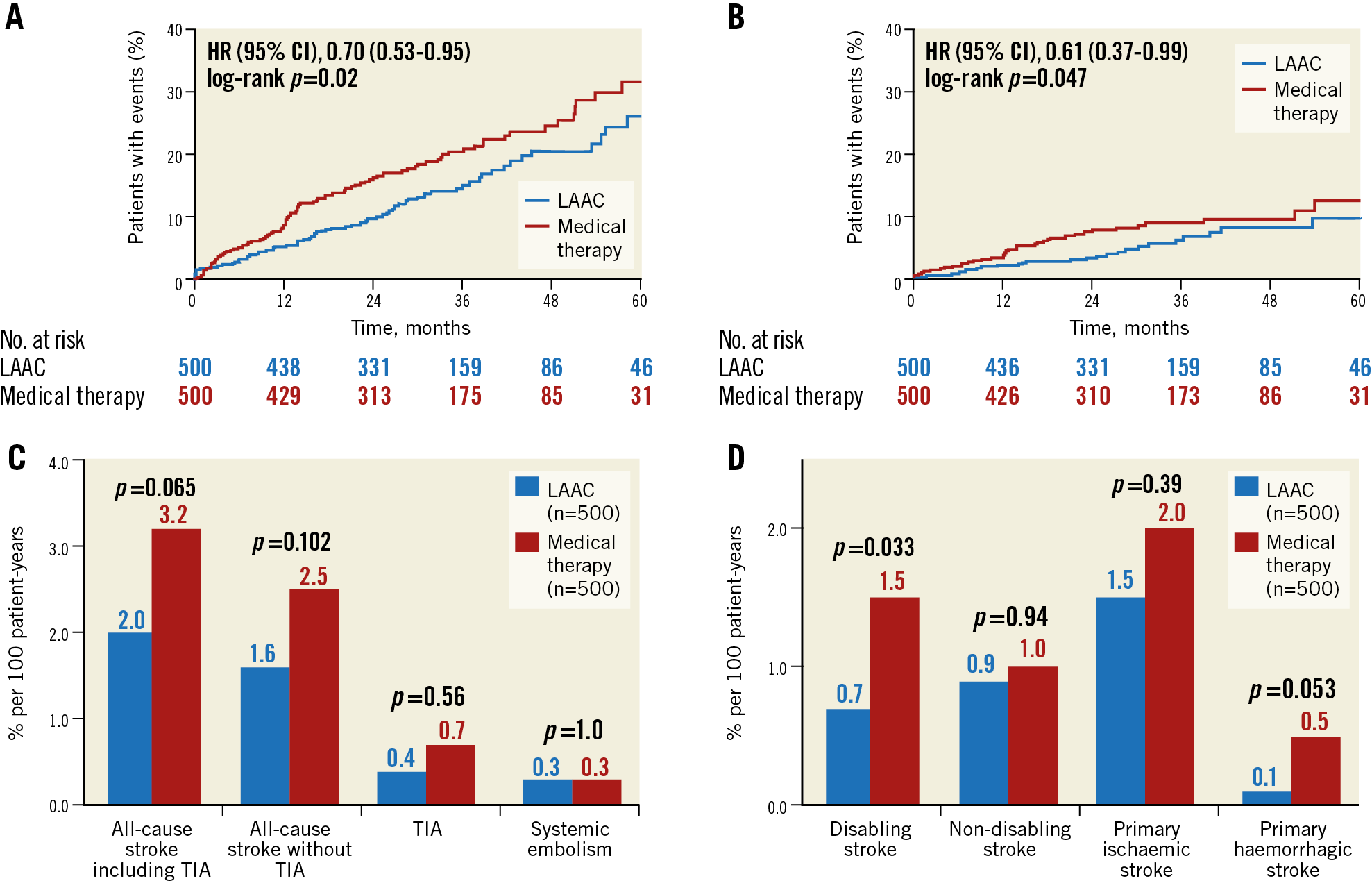
Figure 2. Kaplan-Meier curves of the primary efficacy endpoint (A), all-cause stroke and TIA (B), and cumulative incidence of ischaemic events (C) and stroke types (D). Event rates per 100 patient-years.
PRIMARY SAFETY ENDPOINT
Safety events occurred in 48 of the 500 LAAC patients during 1,342 patient-years, i.e., 3.6%, versus in 60 of the 500 control group patients during 1,303 patient-years, i.e., 4.6% per 100 patient-years (HR 0.80, 95% CI: 0.55-1.18, p=0.21). Of the 48 safety events in the LAAC group, 25 (52.1%) were caused by severe procedural adverse events and 23 (47.9%) by major, life-threatening or fatal bleedings during follow-up (Table 2, Figure 3).
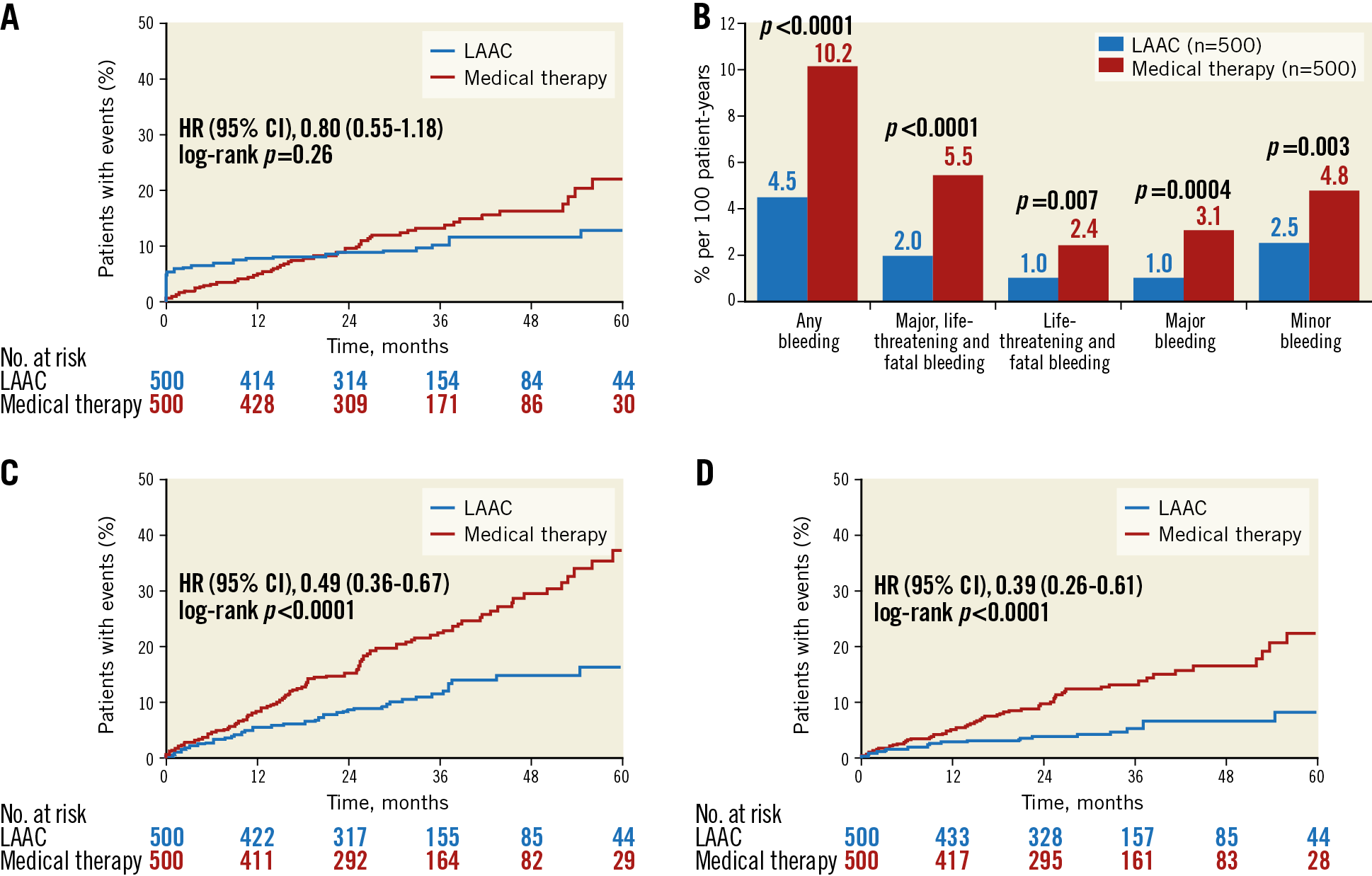
Figure 3. Kaplan-Meier curves of the primary safety endpoint (A) and cumulative incidence of the different types of bleeding (B). Kaplan-Meier curves of any bleeding (C) and major and life-threatening bleedings only (D). Event rates per 100 patient-years.
COMBINED HAZARD ENDPOINT (NET CLINICAL BENEFIT)
The combination of efficacy and safety events occurred in 109 LAAC patients during 1,342 patient-years, i.e., 8.1%, versus in 142 control group patients during 1,303 patient-years, i.e., 10.9% per 100 patient-years (HR 0.76, 95% CI: 0.60-0.97, p=0.018) (Table 2, Figure 4).
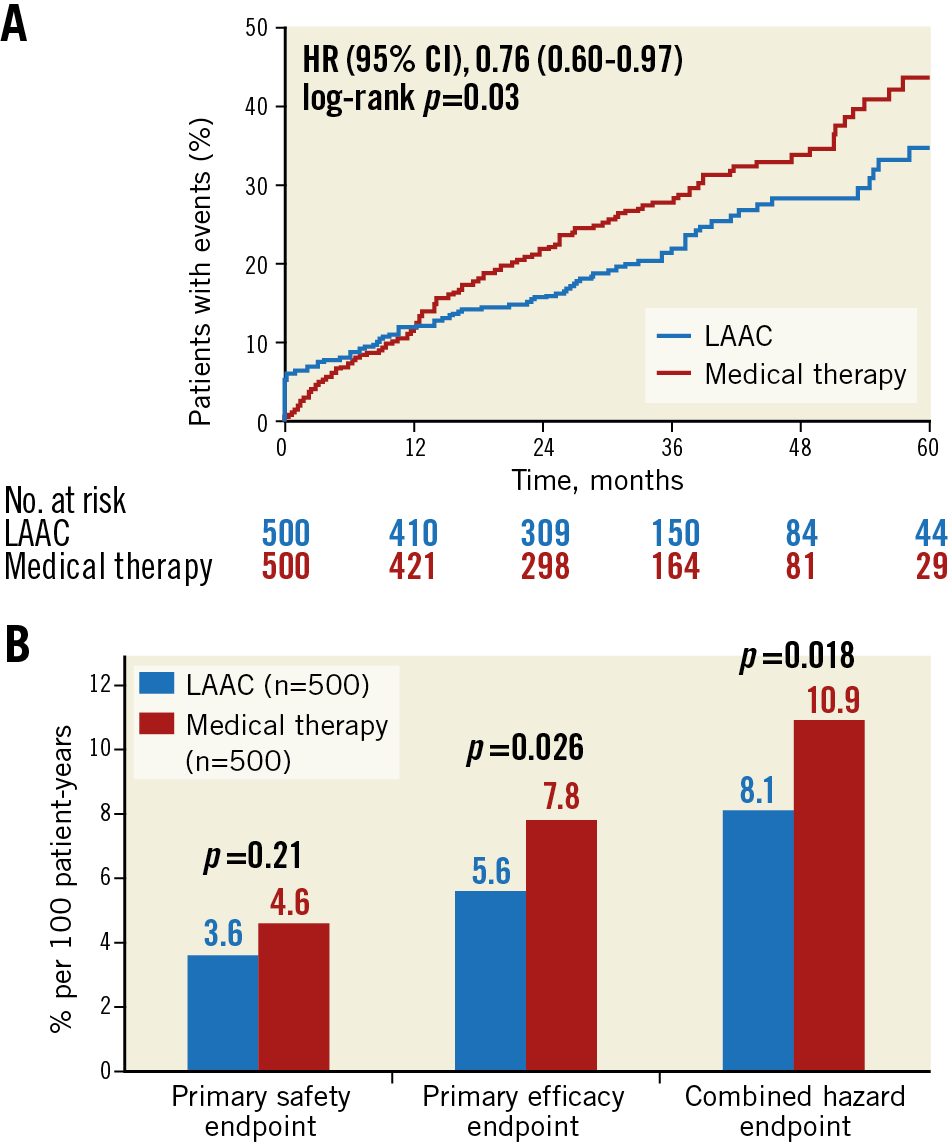
Figure 4. Kaplan-Meier curves of the net clinical benefit (A) and cumulative incidence of event rates per 100 patient-years of the predefined endpoints (B).
MORTALITY
All-cause mortality was lower in the LAAC group (111/1,342, 8.3% vs 151/1,303, 11.6% per 100 patient-years, HR 0.72, 95% CI: 0.56-0.92, p=0.005). It was driven mainly by a lower rate of cardiovascular and unexplained deaths (54/1,342, 4.0% vs 84/1,303, 6.5% per 100 patient-years, HR 0.64, 95% CI: 0.46-0.89, p=0.007), whereas non-cardiovascular mortality was similar between the groups (57/1,342, 4.3% vs 67/1,303, 5.1%, p=0.32) (Supplementary Table 3, Supplementary Figure 2). The causes of death are listed in Supplementary Table 3.
Discussion
In this propensity-matched dual-centre study of 1,000 patients with AF, LAAC with AMPLATZER occluders was compared to medical therapy. After 2,645 patient-years and at a mean follow-up of 2.7±1.5 years, the principal findings were:
1) LAAC with AMPLATZER occluders showed superior efficacy over medical therapy, driven evenly by a lower incidence of cardiovascular mortality and strokes.
2) The composite safety endpoint in the LAAC group was similar to medical therapy and was driven mainly by procedural complications. With regard to non-procedure-related bleedings, LAAC was superior to medical therapy.
3) LAAC with AMPLATZER occluders showed a significant net clinical benefit over medical therapy.
In contrast to LAAC with the WATCHMAN occluder, no randomised controlled data against medical therapy with AMPLATZER occluders have been available so far7,9. Therefore, until definite results of ongoing randomised trials become available22, we performed the APPLY study with a sizeable cohort of 1,000 patients. By PSM, good overall comparability between our all-comer high-risk LAAC cohort and a corresponding high-risk cohort of patients managed with standard medical therapy was shown. In spite of or because of dealing with higher-risk patients, APPLY resembles the results of the WATCHMAN trials, supporting the basic concept of LAAC for prevention not only of stroke but also of death and bleedings, irrespective of the device6,7,9. Consistent with the PROTECT-AF trial, death rates after LAAC were also lower in APPLY, despite the above-mentioned differences in four subcategories of the risk scores, where three of four, i.e., heart failure, prior stroke, and bleedings, were more frequent in the LAAC group. While non-cardiovascular death was similar in both groups, overall mortality was lower in the LAAC group. This was due to the lower rates of cardiovascular and unknown causes of death, which in turn was the result of lower rates of stroke and TIA, a lower rate of disabling strokes, fewer heart failure-related deaths (despite the higher baseline rate in the LAAC group), and presumably fewer clinically occult bleedings (Figure 2, Figure 3, Supplementary Figure 2, Supplementary Table 3).
Given the fact that 99.5% of the patients in the LAAC group of APPLY had some kind of surgical, device-based or, in case of platelet inhibition only, incomplete medical stroke protection, the lower rate of stroke and TIA, as well as the lower rate of disabling strokes and the strong trend towards lower rates of stroke and systemic embolism, is compelling (Figure 2B-Figure 2D). Our rate of 1.9% is comparable to the rate of the five-year outcomes after LAAC with the WATCHMAN of the PREVAIL and PROTECT-AF trials (1.7%) and lower than in the AMPLATZER Amulet global observational registry, where the stroke rate was 2.9% in a very elderly and high-risk cohort of 1,088 patients11. In contrast, only 78.8% of patients in the control group of APPLY were provided with an adequate stroke prophylaxis, reflecting the real-world issues of medically managed high-risk AF patients. Therefore, with time, the gap between the two groups in both efficacy and safety will probably spread further. In contrast to the above-mentioned WATCHMAN trials, not only the overall stroke rate, but also ischaemic stroke rates were lower in APPLY despite a higher stroke risk at baseline (1.5% vs 2.0% in APPLY vs 1.6% and 0.95% in the five-year patient-level meta-analysis of PROTECT-AF and PREVAIL)9. These figures are consistent with current AMPLATZER registries and support the assumption that, due to device-specific reasons, AMPLATZER occluders may be less thrombogenic than the WATCHMAN.
With regard to bleedings, the rates of both groups in APPLY are higher than in the WATCHMAN trials, reflecting our higher-risk cohorts. Due to immediate cessation of anticoagulation directly after LAAC in the vast majority of patients, and in contrast to the WATCHMAN trials, all categories of bleedings were drastically reduced in this group (Table 2, Figure 3). The relatively high rate of patients in the control group who were taking OAC in addition to a single (7.6%) or dual (2.2%) antiplatelet therapy may also explain the higher bleeding rate in this group. On the other hand, 24% of the patients did not take any anticoagulation, which counterbalances this issue. Ischaemia protection suffers from relatively poor compliance with OAC. Compliance is not an issue after LAAC which blunts the assumed inferior protection against ischaemia of LAAC compared to optimal OAC. When added to the fact that bleedings are constantly and independently linked to an increased risk of mortality, the higher rate of cardiovascular/unknown deaths in APPLY in the control group becomes plausible20. This is also consistent with the lower rate of hospital stays due to fewer bleedings and a better self-reported functional status in the LAAC group of APPLY (Supplementary Table 2, Supplementary Figure 1B).
Limitations
A major limitation of APPLY is its retrospective design and the bias that, despite the PSM, the rates for prior bleedings and strokes, heart failure and diabetes mellitus (DM) (the first three in disfavour of the LAAC group, the latter in disfavour of the control group) were different between the groups. On the one hand, the higher DM rate in the control group may have contributed to the higher cardiovascular mortality in this group. On the other hand, this effect is likely to be counteracted by the fact that three strongly prognostically relevant characteristics, namely congestive heart failure and prior stroke and bleeding rates were more prevalent in the LAAC group, where the rates of death due to congestive heart failure and stroke were similar rather than higher (Supplementary Table 3). The fact that already at study inclusion only 76% of patients of the control group left hospital with OAC may be regarded as a limitation, but it reflects the real-world character of APPLY and the well-known problems of any OAC in high-risk patients. A selection of 500 patients suitable for OAC and comparable with our high-risk LAAC cohort probably does not exist. This is a general problem of LAAC in patients receiving AMPLATZER occluders and different from randomised WATCHMAN studies, in which patients had to be eligible for OAC. Finally, and again due to the observational nature of APPLY, drug types in patients who were anticoagulated were heterogeneous, since one third received NOAC at the time of follow-up. Therefore, our results cannot be interpreted specifically for patients receiving either VKA only or NOAC only. The results of the prospective randomised and controlled PRAGUE-17 trial comparing LAAC with AMPLATZER or WATCHMAN occluders with NOAC, mainly with apixaban, are less contrasting, i.e., a significant superiority in non-procedure-related bleedings could not be shown23,24,25. This may be due to a lack of power to determine differences in the single components of the primary endpoint (which was a combined endpoint of stroke, death and bleeding) and by the comparison of LAAC to the predominantly used apixaban with relatively favourable bleeding rates. In a recently published propensity score-matched retrospective study, no differences in efficacy and safety were found between LAAC with WATCHMAN and AMPLATZER occluders (n=96) and various NOAC (n=96)26.
Conclusions
Results from APPLY suggest that left atrial appendage closure with AMPLATZER devices offers a significant net clinical benefit over medical therapy by way of superior efficacy, similar safety and lower all-cause and cardiovascular mortality.
|
Impact on daily practice Prevention of thromboembolic events in patients with AF remains a frequent and often challenging problem, especially in patients with a contraindication to OAC, the most frequently used therapeutic option. In this dual-centre, real-world propensity score-matched study comparing LAAC to medical therapy with OAC, LAAC showed a net clinical benefit compared to OAC, driven by superior efficacy. Thus, LAAC is a valuable treatment option in patients with AF. |
Funding
Funding for APPLY was provided by the Swiss Heart Foundation.
Conflict of interest statement
S. Windecker has received grants to the institution from Abbott, Biotronik, Boston Scientific, Medtronic and Edwards Lifesciences. B. Meier has received speaker and proctor fees from Abbott, Bayer and Sanofi. F. Nietlispach is a consultant to Abbott, Edwards Lifesciences and Medtronic. S. Gloekler has received grants to the institution from Abbott and the Swiss Heart Foundation. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.




