Abstract
It has been speculated that brain activities might directly control adaptive immune responses in lymphoid organs, although there is little evidence for this. Here we show that splenic denervation in mice specifically compromises the formation of plasma cells during a T cell-dependent but not T cell-independent immune response. Splenic nerve activity enhances plasma cell production in a manner that requires B-cell responsiveness to acetylcholine mediated by the α9 nicotinic receptor, and T cells that express choline acetyl transferase1,2 probably act as a relay between the noradrenergic nerve and acetylcholine-responding B cells. We show that neurons in the central nucleus of the amygdala (CeA) and the paraventricular nucleus (PVN) that express corticotropin-releasing hormone (CRH) are connected to the splenic nerve; ablation or pharmacogenetic inhibition of these neurons reduces plasma cell formation, whereas pharmacogenetic activation of these neurons increases plasma cell abundance after immunization. In a newly developed behaviour regimen, mice are made to stand on an elevated platform, leading to activation of CeA and PVN CRH neurons and increased plasma cell formation. In immunized mice, the elevated platform regimen induces an increase in antigen-specific IgG antibodies in a manner that depends on CRH neurons in the CeA and PVN, an intact splenic nerve, and B cell expression of the α9 acetylcholine receptor. By identifying a specific brain–spleen neural connection that autonomically enhances humoral responses and demonstrating immune stimulation by a bodily behaviour, our study reveals brain control of adaptive immunity and suggests the possibility to enhance immunocompetency by behavioural intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data generated here are included within the paper (and its Supplementary Information files) or are available from the corresponding authors upon reasonable request. Source data for Figs. 1–5 and Extended Data Figs. 1–10 are provided with the paper.
References
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Rinner, I. & Schauenstein, K. Detection of choline-acetyltransferase activity in lymphocytes. J. Neurosci. Res. 35, 188–191 (1993).
Sato, K. Z. et al. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci. Lett. 266, 17–20 (1999).
Wessler, I. & Kirkpatrick, C. J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 154, 1558–1571 (2008).
Saalmüller, A. & Mettenleiter, T. C. Rapid identification and quantitation of cells infected by recombinant herpesvirus (pseudorabies virus) using a fluorescence-based β-galactosidase assay and flow cytometry. J. Virol. Methods 44, 99–108 (1993).
Cano, G., Sved, A. F., Rinaman, L., Rabin, B. S. & Card, J. P. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol. 439, 1–18 (2001).
Keifer, O. P. Jr, Hurt, R. C., Ressler, K. J. & Marvar, P. J. The physiology of fear: reconceptualizing the role of the central amygdala in fear learning. Physiology 30, 389–401 (2015).
Herman, J. P. & Tasker, J. G. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front. Endocrinol. 7, 137 (2016).
Peng, J. et al. A quantitative analysis of the distribution of CRH neurons in whole mouse brain. Front. Neuroanat. 11, 63 (2017).
Kadmiel, M. & Cidlowski, J. A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 34, 518–530 (2013).
Vandevyver, S., Dejager, L., Tuckermann, J. & Libert, C. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology 154, 993–1007 (2013).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Ben-Shaanan, T. L. et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944 (2016).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Lightman, S. L. & Conway-Campbell, B. L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11, 710–718 (2010).
Ulrich-Lai, Y. M. & Herman, J. P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (2009).
Wang, Y. et al. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat. Immunol. 18, 921–930 (2017).
Kruse, A. C. et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013).
Li, Y. et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat. Commun. 7, 10503 (2016).
Paxinos, G. & Franklin, K. B. J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates 4th edn (Academic, 2012).
Acknowledgements
We thank S. Chavan for sharing technical information about electrophysiological recording. The work was funded in part by the National Key R&D Program of China (Ministry of Science and Technology, 2018YFE0200300 to H.Q.), National Natural Science Foundation of China (grants 81621002, 31830023 to H.Q.; grants 61890951, 61890950, 31671086 to J.H.), the Tsinghua-Peking Center for Life Sciences, and the Beijing Municipal Science & Technology Commission.
Author information
Authors and Affiliations
Contributions
H.Q. conceptualized and supervised the study. X.Z. developed the splenic denervation technique and, together with L.Z., the EPS regimen. X.Z., B.L. and Y.Y. participated in designing the overall study together with H.Q., J.H., and Y.Z. X.Z. and L.Z. conducted most of the immunological experiments. L.H. synthesized the acetylcholine analogue under the supervision of X.L. Y.Y., S.J., and L.Z. conducted the PRV tracing experiments under the supervision of F.X. and J.H. X.Z. and Y.Y. conducted electrophysiological recording of the splenic nerve. X.Z., Y.Y. and L.Z. conducted CRH neuron ablation and fibre photometry studies under the supervision of J.H., W.S. and H.Q. B.L., X.Z., B.K. and L.Z. conducted CRH neuron inhibition and activation studies under the supervision of Y.Z. and H.Q. All authors contributed to data interpretation. H.Q. and X.Z. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Jonathan Kipnis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
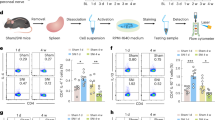
Extended Data Fig. 1 Surgical splenic denervation and its lack of effects on plasma cell formation in a T-independent response.
a–c, Photographs of surgical exposure and alcohol application for denervation or sham operation. See Methods for detailed description. d, e, Whole spleen cross-sections from sham-operated (d) and denervated (e) mice, from which the images in Fig. 1a were cropped (yellow boxes). f, g, Representative images of whole spleen sections of sham-operated (f) or denervated (g) mice 1 week after surgery. Data representative of two experiments. Green, TH staining; blue, IgD staining of follicle; red, CD3ε staining of T-cell zone. h, Norepinephrine (NE) concentrations in splenic tissue from sham-operated and denervated mice 13 days after NP-KLH immunization. Data pooled from three experiments. Each symbol denotes one mouse, lines denote means. i, Representative contour plots (left) and summary apoptotic frequencies (right) in CD4 T and B cell compartments of sham and denervated mice. Each symbol denotes one mouse, lines denote means. j, k, Percentage SPPC 7days (j) or 13 days (k) after NP-Ficoll immunization in sham and denervated mice. Representative FACS contour plots (left) and summary data in scatter plots (right). Data pooled from two independent experiments, with each symbol indicating one mouse; lines denote means. Two-tailed unpaired t-test (h–k).
Extended Data Fig. 2 Effects of acetylcholine on plasma cell formation.
a, b, Percentage SPPC (gated as in Fig. 1d) and GC (gated as in Fig. 1c) 13 days after NP-KLH immunization in mice that were injected subcutaneously with acetylcholine chloride (a) or noradrenaline bitartrate monohydrate (b) at the indicated doses twice daily from day 8 to day 12 after immunization. Data pooled from three independent experiments; each symbol indicates one mouse, lines denote means. One-way ANOVA. c, d, SPPC formation. c, Representative contour plots showing gated plasma cells in donor CD45.1 B cells that were transduced (GFP+) with the AchE-expressing, control, or COMT-expressing vector (top row), donor CD45.1 B cells that were not transduced (GFP−) (middle row), or recipient B cells of the same recipient host (CD45.2) after NP-KLH immunization (bottom row). d, Summary statistics of percentate SPPC in transduced donor B cells (top), non-transduced donor B cells (middle) and recipient B cells (bottom). Data were pooled from three independent experiments. Each symbol indicates one mouse, lines indicate means. All transduction efficiencies were 10–15%. Two-sided unpaired t-test.
Extended Data Fig. 3 Generation of mice deficient in AChRs and detection of acetylcholine binding by acetylcholine analogue.
a–d, Schematic of Chrna9 exon 4 (a) and Chrnb4 exon 5 (c), containing transmembrane domain-coding sequences, with relative positions of the targeting guide RNAs (sg1, sg2) and the genotyping primers. DNA sequences of relevant Chrna9 (a) or Chrnb4 (c) gene segments, with the yellow highlight indicating deleted sequences and the red highlight indicating stop codons resulting from the frameshift. PCR genotyping results of Chrna9 (b) or Chrnb4 (d) wildtype (WT) and knockout (KO) alleles. e, f, Bone-marrow B cell development (e) and splenic marginal zone B cell development (f) in Chrna9+/+ and Chrna9−/− mice. Representative FACS contour plots and summary data in scatter plots from one of two experiments. Each symbol indicates one mouse, lines indicate means. Two-sided unpaired t-test. g, Reaction scheme for synthesis of the acetylcholine analogue, 2-(2-azidoacetoxy)-N,N,N-trimethylethan-1-aminium bromide (III). See Methods for details. h, i, Comparison of acetylcholine-binding capacities of wild-type GC, SPPC and total B cells (h) and comparison of acetylcholine-binding capacities of Chrna9+/+ and Chrna9−/− cells of indicated types (i), as measured by staining with the acetylcholine analogue, chemical III. One of three experiments with similar results is shown.
Extended Data Fig. 4 Requirement for acetylcholine receptors for optimal SPPC formation.
a, μMT mice were reconstituted by intravenous transfer of wild-type, Chrna9−/− or Chrnb4−/− mature B cells one week before NP-KLH immunization, and percentage SPPC was measured 13 days after immunization. b, Representative contour plots and summary statistics of percentage SPPC in reconstituted μMT mice following immunization, as described in a. Data pooled from three independent experiments, two of which had wild-type, Chrna9−/− and Chrnb4−/− donors and one of which had no Chrnb4−/− group. Points denote individual mice, lines denote means. Two-sided unpaired t-test. c, The protocol to interrogate the dependence of Chrna9 effects on splenic nerve, as used in Fig. 2d, e.
Extended Data Fig. 5 Involvement of T cells but not B cells as a potential source of acetylcholine for promoting SPPC formation.
a, The protocol for examining the contribution and function of ChAT+ CD4+ T cells. b, Relative levels of Chat mRNA in ChAT+ T cells (CD4+CD44+TdTomato+) and ChAT− T cells (CD4+CD44+TdTomato−) sorted from Tcrb−/−Tcrd−/− knockout mice that were reconstituted with intravenously infused ChAT-IRES-Cre × Rosa26-Ai14 mature T cells and immunized with NP-KLH at day 8. Data were pooled from two independent experiments, with each symbol indicating one independent sort and lines indicating means. c, Colocalization (yellow circles) of some ChAT+ T cells (red), splenic nerve fibres (TH+, green) and aggregates of SPPCs (Igκhigh, white) on splenic tissue sections taken 10 days after NP-KLH immunization. Representative of two experiments. Scale bar, 200 μm. d, e, Representative contour plots (d) and summary data (e) of percentages of SPPC and GC in DT-treated mice of the indicated genotypes by the protocol shown in a. Data pooled from four independent experiments with each symbol indicating one mouse and the lines denoting the means. f, The protocol for examining the contribution and function of ChAT+ B cells. g, h, Representative contour plots (g) and summary data (h) of percentages of SPPC and GC in DT-treated mice of the indicated genotypes by the protocol shown in f. Each symbol indicates one mouse from two independent experiments, and lines denote means. Two-sided unpaired t-test (b, e, h).
Extended Data Fig. 6 Retrograde PRV tracing from the spleen and setup for splenic nerve recording.
a–d, Selected CNS regions that were labelled by PRV. Scale bars, 50 μm. PSth, parasubthalamic nucleus; LC, locus coeruleus; SubC, subcoeruleus nucleus; NTS, nucleus tractus solitarius; VLM, ventrolateral medulla; LPGi, lateral paragigantocellular nucleus. e–g, Setup for optogenetic CRH neuron stimulation and splenic nerve recording in CRH-IRES-Cre mice. e, Procedural diagram for recording splenic nerve activities during optogenetic activation of CRH neurons in the CeA and PVN. f, Optical fibre implantation to the PVN and CeA regions in the brain. Red, ChR2+ neurons. Scale bars, 50 μm. g, Electrode implantation around the splenic nerve. Data are representative of two independent experiments.
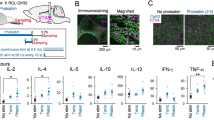
Extended Data Fig. 7 Efficiency of ablation of CRH neurons and functional verification of DREADD chemogenetics.
a–c, The efficiency of CRH neuron ablation. a, Representative coronal sections containing CeA and PVN regions from CRH-IRES-Cre:Rosa26Ai3 mice sham-injected (Control) or injected with AAV-caspase 3 (Ablation) into the CeA and PVN (boxes). b, Magnified views of the boxed areas in a. Scale bars, 50 μm. c, The relative abundance of Ai3+ cells in the PVN and CeA in control (green) or CRH-ablated mice (open bars), with cell numbers in the control group set as 1. Data from four pairs of mice in four independent experiments. Two-sided unpaired t-test. d, e, Validation of hM4D(Gi)-mediated inhibition (d) and hM3D(Gq)-mediated activation (e) by CNO in brain slices. Top, representative recordings from single CRH-IRES-Cre neurons that expressed hM4D(Gi)–mCherry for inhibition (d) or hM3D(Gq)–mCherry for activation (e) (AAV details in Fig. 3b). Bottom, firing rates of single hM4D(Gi)- or hM3D(Gq)-expressing CRH neurons from indicated regions, before and after perfusion of the brain slices with CNO (5 μM). Each line with connected symbols indicates one neuron before and after CNO; data collected from three mice in two experiments. Two-sided paired t-test.
Extended Data Fig. 8 CRH neuron-mediated promotion of plasma cell formation requires the splenic nerve.
a, A schematic diagram of the experimental setup. b, The gating strategy for NP-specific IgG+ SPPCs, from a parental gate of B-lineage cells as in Fig. 1b. c, Batch-normalized NP-specific percentage SPPC in relative units (R.U.). For each experiment, the average SPPC% in the Sham–eYFP group is set as unit 1, against which percentage SPPC in individual mice of the four groups in the same experiment was normalized to obtain the R.U. for each animal. R.U. data are pooled from three independent experiments. Each symbol denotes one mouse, and lines indicate means. Two-sided unpaired t-test. YFP, AAV-DIO-eYFP group, 3Q, AAV-DIO-hM3D(Gq) group.
Extended Data Fig. 9 Distinct stress and immunomodulatory effects of EPS and PPR.
a, Diagram of the EPS setup. b, GCaMP6m expression in the PVN (top) or CeA (bottom) of CRH-IRES-Cre mice as the result of stereotactic AAV injection and positions of implanted optic fibres, outlined in boxes, for photometric measurement of calcium signals in the respective regions. Scale bar, 100 μm. c, Representative traces of integrated calcium signals, presented as normalized changes in the GCaMP6m fluorescence intensity (mean in red, s.e.m. in grey), from CRH neurons in the PVN (top) or CeA (bottom) of CRH-IRES-Cre mice undergoing EPS and then PPR, 3 days apart. Dashed lines mark the beginning and end of behavioural regimens. d, Average GCaMP6m signals during EPS and PPR sessions. Each line denotes one mouse; column heights and error bars show mean ± s.e.m. of six mice. Two-sided paired t-tests. e, The schedule of collecting blood samples from mice subjected to EPS (two collection points as indicated) or PPR for corticosterone measurement. f, Normalized serum corticosterone levels. One-way ANOVA with Bonferroni’s correction. g, Representative contour plots (left) and summary statistics of percentage SPPC and GC (right), 13 days after immunization with NP-KLH in mice that were untreated or subjected to PPR twice daily between day 8 and day 12. Data pooled from four independent experiments, with each symbol indicating one mouse and lines indicating means. Two-sided unpaired t-test.
Extended Data Fig. 10 A replication study of the effects of EPS on humoral immunity over 4 weeks.
a, Schematic of the experiment setup. b, NP-specific IgG in control and EPS-treated mice, quantified as the dilution factor at EC50, calculated for individual mice as in Fig. 5. Each symbol denotes one mouse (17 untreated, 16 EPS), and lines indicate means. c, Mutations per VH186.2 heavy chain in SPPCs on day 13. Each symbol represents one unique VH186.2 sequence recovered, and lines indicate means. d, e, Gating strategy for (d) and frequencies of (e) NP-binding B220−CD138+IgM− plasma cells in the bone marrow. Each symbol represents one mouse, and lines indicate means. Data pooled from three experiments. Two-sided unpaired Mann–Whitney test (b, c) or t-test (e).
Supplementary information
Supplementary Information
This file contains a detailed discussion of results presented in Extended Data Figure 2, Supplementary References and Supplementary Tables 1-3.
Video 1
A 3 minute recording of typical behaviours of a mouse undergoing EPS.
Source data
Rights and permissions
About this article
Cite this article
Zhang, X., Lei, B., Yuan, Y. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020). https://doi.org/10.1038/s41586-020-2235-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2235-7
This article is cited by
-
Somatosensory cortex and central amygdala regulate neuropathic pain-mediated peripheral immune response via vagal projections to the spleen
Nature Neuroscience (2024)
-
Stress increases hepatic release of lipocalin 2 which contributes to anxiety-like behavior in mice
Nature Communications (2024)
-
Effects of lifestyle factors on leukocytes in cardiovascular health and disease
Nature Reviews Cardiology (2024)
-
Interorgan communication networks in the kidney–lung axis
Nature Reviews Nephrology (2024)
-
The cancer-immune dialogue in the context of stress
Nature Reviews Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.