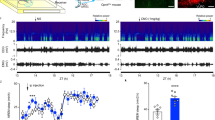
Abstract
Daily changes in light and food availability are major time cues that influence circadian timing1. However, little is known about the circuits that integrate these time cues to drive a coherent circadian output1,2,3. Here we investigate whether retinal inputs modulate entrainment to nonphotic cues such as time-restricted feeding. Photic information is relayed to the suprachiasmatic nucleus (SCN)—the central circadian pacemaker—and the intergeniculate leaflet (IGL) through intrinsically photosensitive retinal ganglion cells (ipRGCs)4. We show that adult mice that lack ipRGCs from the early postnatal stages have impaired entrainment to time-restricted feeding, whereas ablation of ipRGCs at later stages had no effect. Innervation of ipRGCs at early postnatal stages influences IGL neurons that express neuropeptide Y (NPY) (hereafter, IGLNPY neurons), guiding the assembly of a functional IGLNPY–SCN circuit. Moreover, silencing IGLNPY neurons in adult mice mimicked the deficits that were induced by ablation of ipRGCs in the early postnatal stages, and acute inhibition of IGLNPY terminals in the SCN decreased food-anticipatory activity. Thus, innervation of ipRGCs in the early postnatal period tunes the IGLNPY–SCN circuit to allow entrainment to time-restricted feeding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The principal data supporting the findings of this Article are available within the figures and the Supplementary Information; additional data that support the findings of this study are available from the corresponding authors on request.
References
Golombek, D. A. & Rosenstein, R. E. Physiology of circadian entrainment. Physiol. Rev. 90, 1063–1102 (2010).
Cappe, C., Rouiller, E. M. & Barone, P. in The Neural Bases of Multisensory Processes (eds Murray, M. M. & Wallace, M. T.) Chapter 2 (CRC, 2012).
Mahoney, J. R. et al. Keeping in touch with the visual system: spatial alignment and multisensory integration of visual-somatosensory inputs. Front. Psychol. 6, 1068 (2015).
Güler, A. D. et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453, 102–105 (2008).
Hattar, S., Liao, H. W., Takao, M., Berson, D. M. & Yau, K. W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
Berson, D. M., Dunn, F. A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002).
Saderi, N. et al. The NPY intergeniculate leaflet projections to the suprachiasmatic nucleus transmit metabolic conditions. Neuroscience 246, 291–300 (2013).
Moore, R. Y. & Card, J. P. Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. J. Comp. Neurol. 344, 403–430 (1994).
Morin, L. P. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol. 243, 4–20 (2013).
Wams, E. J., Riede, S. J., van der Laan, I., ten Bulte, T. & Hut, R. A. in Biological Timekeeping: Clocks, Rhythms and Behaviour (ed. Kumar, V.) 395–404 (Springer India, 2017).
Chew, K. S. et al. A subset of ipRGCs regulates both maturation of the circadian clock and segregation of retinogeniculate projections in mice. eLife 6, e22861 (2017).
Mistlberger, R. E. Food-anticipatory circadian rhythms: concepts and methods. Eur. J. Neurosci. 30, 1718–1729 (2009).
Acosta-Galvan, G. et al. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc. Natl Acad. Sci. USA 108, 5813–5818 (2011).
Patton, D. F. et al. Photic and pineal modulation of food anticipatory circadian activity rhythms in rodents. PLoS ONE 8, e81588 (2013).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Camiña, J. P. et al. Regulation of ghrelin secretion and action. Endocrine 22, 5–12 (2003).
Blum, I. D., Lamont, E. W., Rodrigues, T. & Abizaid, A. Isolating neural correlates of the pacemaker for food anticipation. PLoS ONE 7, e36117 (2012).
Glass, J. D., Guinn, J., Kaur, G. & Francl, J. M. On the intrinsic regulation of neuropeptide Y release in the mammalian suprachiasmatic nucleus circadian clock. Eur. J. Neurosci. 31, 1117–1126 (2010).
Andermann, M. L. & Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Chen, S.-K., Badea, T. C. & Hattar, S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 476, 92–95 (2011).
Fernandez, D. C. et al. Light affects mood and learning through distinct retina–brain pathways. Cell 175, 71–84.e18 (2018).
Sindelar, D. K., Palmiter, R. D., Woods, S. C. & Schwartz, M. W. Attenuated feeding responses to circadian and palatability cues in mice lacking neuropeptide Y. Peptides 26, 2597–2602 (2005).
May, P. J. The mammalian superior colliculus: laminar structure and connections. Prog. Brain Res. 151, 321–378 (2006).
Gandhi, N. J. & Katnani, H. A. Motor functions of the superior colliculus. Annu. Rev. Neurosci. 34, 205–231 (2011).
Stein, B. E., Stanford, T. R. & Rowland, B. A. Development of multisensory integration from the perspective of the individual neuron. Nat. Rev. Neurosci. 15, 520–535 (2014).
Mundiñano, I. C. & Martínez-Millán, L. Somatosensory cross-modal plasticity in the superior colliculus of visually deafferented rats. Neuroscience 165, 1457–1470 (2010).
Waterson, M. J. & Horvath, T. L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 22, 962–970 (2015).
Sheng, Z., Santiago, A. M., Thomas, M. P. & Routh, V. H. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell. Neurosci. 62, 30–41 (2014).
Azevedo, E. P. et al. A role of Drd2 hippocampal neurons in context-dependent food intake. Neuron 102, 873–886.e5 (2019).
Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 15, 393–405 (2019).
Pendergast, J. S. & Yamazaki, S. The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J. Biol. Rhythms 33, 458–474 (2018).
Acosta-Rodríguez, V. A., de Groot, M. H. M., Rijo-Ferreira, F., Green, C. B. & Takahashi, J. S. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 26, 267–277.e2 (2017).
Franklin, K. B. J. & Paxinos, G. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates (Academic, 2013).
Charan, J. & Kantharia, N. D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 4, 303–306 (2013).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Acknowledgements
We thank the members of the SLCR at NIMH, the Johns Hopkins Biology Mouse Tri-Lab and M. E. Mercau for helpful discussions; O. Gavrilova and the NIDDK Mouse Metabolism Core for their skillful technical assistance; and V. Acosta-Rodríguez for her assistance with the programmable feeders. This work was supported by the NIH (GM076430, EY027202), the generous contributions of the PEW Charitable Trusts (to D.C.F.) and the intramural research fund at the National Institute of Mental Health (ZIAMH002964-02).
Author information
Authors and Affiliations
Contributions
D.C.F. contributed to conceptualization, formal analysis, investigation, methodology, project administration, supervision, visualization and writing (original draft and editing); R.K., J.L. and J.M. contributed to investigation, methodology and writing (reviewing and editing). P.Q.D. contributed to investigation and methodology. M.P. and H.Z. contributed to funding acquisition and writing (review and editing). S.H. contributed to conceptualization, funding acquisition, project administration, supervision and writing (original draft and editing).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Joseph Bass, Sarah Chellappa, Frank Scheer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Early ablation of ipRGCs alters entrainment to TRF.
a, b, General locomotor activity and feeding behaviour were monitored using infrared sensors and programmable feeders, respectively. Representative actograms obtained from an Opn4DTA mouse under free-running (constant darkness and ad libitum access to food) are shown (a). Periodograms were obtained, and no differences in period lengths were found between locomotor activity and the feeding behaviour for both groups (b). Data are mean ± s.e.m. (n = 12 mice for each genotype), two-way ANOVA, followed by Sidak’s multiple comparisons test. c, d, Representative actograms obtained from control (c) and Opn4DTA (d) mice exposed to TRF are shown. e, Under constant darkness and ad libitum acess to food, Opn4DTA mice displayed different free-running periods. Therefore, we analysed whether there is any correlation between the food-anticipatory activity (measured during a 3-h time window) and the time difference (measured in minutes) between the onset of locomotor activity and the time of food delivery measured during the first day of food restriction. No significant correlations (Pearson correlation test, P = 0.6379) were observed in Opn4DTA mice (n = 13 mice). f, The daily total amount of food consumed was measured in male (M) and female (F) control and Opn4DTA mice exposed to the free-running (ad libitum access to food) or TRF paradigm. Data are mean ± s.e.m. (n = 8 mice for each genotype), two-way ANOVA, followed by Sidak’s multiple comparisons test. g, Total food consumption per day during TRF. Data are mean ± s.e.m. (n = 8 mice for each genotype), multiple Student’s t-test, two tailed. h, Pattern of food consumption during the 7 h of food access. Data are mean ± s.e.m. (n = 8 mice for each genotype); multiple Student’s t-test, two tailed. i, Measurement of the body weight of female and male mice exposed to the TRF paradigm. Data are mean ± s.e.m. (n = 12 mice for each genotype), two-way ANOVA, followed by Sidak’s multiple comparisons test. j, The body composition was measured in mice with ad libitum access to food (AL), or on the 21st day of TRF. Data are mean ± SEM (n = 8 mice for each genotype), two-way ANOVA, followed by Sidak’s multiple comparisons test. k, Total ghrelin levels (ng ml−1) were measured in control and Opn4DTA mice after 7, 14, and 21 days of TRF. In all cases, samples were collected immediately before food delivery. Data are mean ± SEM (n = 8 mice for each genotype), Student’s t-test, two tailed.
Extended Data Fig. 2 Early ablation of ipRGCs causes alterations in the IGLNPY–SCN circuit.
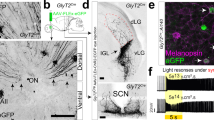
a–c, FOS induction in the arcuate nucleus mediated by the expected food access. Mice were exposed to TRF and perfused on the 21st day at the expected food time (immediately before food delivery). As controls, mice with ab libitum access to food were perfused at circadian time 12. All mice were housed under constant darkness. The area analysed is shown in a diagram of a representative coronal brain section (a). Representative images of control and Opn4DTA mice exposed to TRF are shown (b); the number of FOS+ cells in the arcuate nucleus was quantified (c). Data are mean ± s.e.m. (n = 4 mice for each genotype), two-tailed Tukey’s tes,. d–h, Projection pattern of IGLNPY cells. Npycre/+ mice were unilaterally injected in the IGL using a Cre-dependent AAV encoding tdTomato (AAV2/9-phSyn1(S)-Flex-tdTomato-T2A-SynEGFP-WPRE). IGLNPY neurons send dense and bilateral projections to the SCN (d) and, to a least extent, unilateral projections to other brain targets, including the dorsal geniculate and dorsal thalamus (e and f, respectively), and the superior colliculus (g). The complete pattern of IGLNPY projections is shown in a diagram of representative coronal brain sections (h). Three independent experiments were performed with similar results. i, Representative SCN sections obtained from control and Opn4DTA mice are shown. Marked alterations in the pattern of NPY staining in the SCN were observed in Opn4DTA mice, compared to control mice. j, Correlation between NPY level in somas and axonal terminals, measured in the IGL and SCN, respectively. Results obtained from control and Opn4DTA mice are shown. Pearson r values were measured for both groups (control = 0.728; Opn4DTA = 0.389). A linear regression was applied, and the comparison of slope fits was not significantly different (slope ± s.e., control = 0.136 ± 0.052; Opn4DTA = 0.100 ± 0.096). The asymptotic normal 95% confidence interval is shown for both groups. (n =7 control mice, 8 Opn4DTA mice). k, Brain targets that are not innervated by ipRGCs and that express NPY were studied in control and Opn4DTA mice. No obvious changes in NPY expression levels were observed in the paraventricular hypothalamic nucleus, arcuate nucleus, paraventricular nucleus of the thalamus or hypothalamic dorsomedial nucleus. Three independent experiments were performed with similar results. CL, centrolateral nucleus of the thalamus; LA, lateroanterior hypothalamic nucleus; LHb, lateral habenula LP, lateral posterior thalamic nucleus; SO, supraoptic nucleus. Scale bar, 100 μm (b, i, k), 200 μm (d, f), 400 μm (e, g).
Extended Data Fig. 3 ipRGCs influence the IGL–SCN circuit during early postnatal stages.
a, b, Representative actograms obtained from Opn4attnnDTA mice housed under TRF (a). A score analysis was performed for all actograms obtained (b). Data are mean ± s.e.m. (n = 6 control mice, 8 Opn4attnDTA mice), Student’s t non-parametric (Mann–Whitney) test, two-tailed. c, Representative coronal sections obtained from wild-type control and enucleated mice are shown. NPY+ fibres were measured in the SCN area. Five independent experiments were performed with similar results. d–g, Representative actograms obtained from wild-type mice enucleated at P0 (d), P40 (e) or P90 (f) under TRF. A score analysis was performed for all actograms obtained (g). Data are mean ± SEM (n = 5 control, 6 En P0, 11 En P40 and 8 En P90 mice), Student’s t non-parametric (Mann–Whitney) test, two-tailed. Scale bar, 100 μm.
Extended Data Fig. 4 Role of SCN-projecting ipRGCs in nonphotic entrainment.
a–c, Retinal innervation (CTB) (red) and NPY staining (green) in the IGL and SCN in control and Opn4cre/+ Brn3bDTA/+ mice. Representative coronal sections are shown (a). NPY staining in the IGL (b) and SCN (c) were analysed in three-month-old mice. Data are mean ± SEM (n = 5 mice for each genotype), two-tailed Student’s t-test. d–h, Control and Opn4cre/+ Brn3bDTA/+ mice were exposed to TRF. Representative actograms are shown (d). The locomotor activity before food access (e) and the food-anticipatory activity (f) were measured. Data are mean ± s.e.m. (n = 7 control mice, 12 Opn4cre/+ Brn3bDTA/+ mice), by two-tailed Student’s t-test. A score analysis was performed for all actograms obtained (g). Data are mean ± s.e.m. (n = 7 control mice, 18 Opn4cre/+ Brn3bDTA/+ mice), by Student’s t non-parametric (Mann–Whitney) test, two-tailed. Representative actograms obtained from Opn4cre/+ Brn3bDTA/+ mice under TRF (h). Scale bars, 100 μm (a, bottom), 200 μm (a, top).
Extended Data Fig. 5 IpRGC input to SCN during early postnatal stages influences afferent IGLNPY projections.
a, Npycre/+ mice, with or without bilateral enucleation at P0, were injected in the IGL with Cre-dependent AAV-tdT-Syn-GFP. b, Representative IGL injections in adult control and P0 enucleated Npycre/+ mice are shown. Three independent experiments were performed with similar results. c, d, Axonal projections (tdTomato) (red) from IGLNPY cells and their synaptic terminals (syn–GFP) (green) are shown (c). IGLNPY synaptic terminals were quantified (d). Data are mean ± s.e.m. (n = 3 mice for each condition), two-tailed Student’s t-test. Scale bars, 200 μm (b), 100 μm (c).
Extended Data Fig. 6 NPY signalling in the IGL–SCN circuit controls nonphotic entrainment.
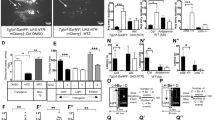
a–d, Representative actograms obtained from NPY-knockout (KO) (Npycre/cre) mice exposed to TRF are shown (a). The locomotor activity 9 h before food access (b) and the food-anticipatory activity (c) were measured for control and NPY-knockout mice. Data are mean ± s.e.m. (n = 7 control mice, 11 NPY-knockout mice), two-tailed Student’s t-test. Additionally, a score analysis was performed for all actograms obtained (d). Data are mean ± s.e.m. (n = 7 control mice, 11 NPY-knockout mice), Student’s t non-parametric (Mann–Whitney) test, two-tailed. In addition, a second mouse line (Npytm1Rpa) was used to evaluate the effects of NPY ablation. Results obtained from both NPY-knockout mouse lines were indistinguishable (data not shown). e, The daily total amount of food consumed (during ad libitum access to food and TRF) was measured in control, NPY-knockout (Npycre/cre), and Npycre/+ mice bilaterally injected in the IGL with a control AAV (AAV5/Syn-DIO-hChR2(H134R)-EGFP-WPRE-HGHpA) or AA encoding TenT (pAAV5/CMV-DIO-eGFP-2A-TeNT). Under free-running conditions (constant darkness and ad libitum access to food), the amount of food consumed by control and NPY-knockout mice (P = 0.0267), as well as control and Npycre/+ mice (P = 0.008), was significantly different. Data are mean ± s.e.m.(n = 5 mice for each condition), two-way ANOVA, followed by Sidak’s multiple comparisons test. f, g, Representative actograms obtained from Npycre/+ mice exposed to TRF and injected with control AAV (f) or AAV encoding TenT (g) are shown.
Extended Data Fig. 7 Neural silencing of the IGLNPY–SCN circuit.
a, Sites of injections (IGL) and optical fibre implantation (SCN) were confirmed at the end of the experiments. b Npycre/+ sham (AAV/DIO-tdTomato) and Npycre/+ ArchT (AAV5/DIO-ArchT-tdTomato) mice were housed under TRF for 3 weeks and the locomotor activity was then measured for 3 h before food access. Results are shown as relative activity (total counts per hour) during food-anticipatory activity. Data are mean ± s.e.m. (n = 5 mice for each condition), two-way ANOVA, followed by Sidak’s multiple comparisons test. c, d, Food consumed during TRF. The average amount of food consumed was measured for 10 days before optogenetic stimulation (before); the amount of food consumed the day of neural silencing was also measured (after) for both groups of mice. Data are mean ± s.e.m. (n = 5 mice for each condition), paired Student’s t-test, two-tailed. e–h, Locomotor activity was quantified (in 5-min bins) starting 3 h before food access. Two hours before food access, neural silencing was induced by applying 3 pulses of light, of 20 min each (shown in green), with 20-min intervals (shown in grey). Results of locomotor activity obtained for all Npycre/+ sham (e) and Npycre/+ ArchT (f) mice are shown. The average of results is also shown (g); similar food-anticipatory activity was observed in both groups without optical stimulation, whereas a significant reduction in locomotor activity was found in Npycre/+ ArchT mice during optogenetic stimulation. Data are mean ± s.e.m. (n = 5 mice for each condition), two-way ANOVA, followed by Sidak’s multiple comparisons test. For Npycre/+ sham mice, no changes in total activity were observed during light stimulation (light), or intervals between light pulses (interval), compared with activity measured during the first hour of food-anticipatory activity (before) (h). We observed a significant reduction in activity with light stimulation compared with interval periods, suggesting that the light could have a direct masking effect on mouse activity. However, the activity was fully recovered immediately after stimulation. Npycre/+ ArchT mice displayed similar and reduced activity for both stimulation and interval periods, compared with the activity displayed before optogenetic stimulation. Data are mean ± s.e.m. (n = 5 mice for each condition), two-tailed Tukey’s test. Scale bars, 100 μm (a, bottom), 200 μm (a, top).
Extended Data Fig. 8 Putative model for the circuit that drives circadian food-anticipatory activity.
Time-restricted access to food constitutes a strong environmental cue that causes the alignment of the circadian system to feeding schedules, driving food-anticipatory activity that precedes the expected meal. The current view in the field is that a widespread system—composed of elements referred to as food-entrainable oscillators (FEOs)—controls the physiological and behavioural responses to TRF. Among the candidates for FEOs are areas of the hypothalamus (such as the paraventricular nucleus, ventromedial hypothalamic nucleus, dorsomedial hypothalamic nucleus and arcuate nucleus), thalamic areas (such as the paraventricular thalamus and the IGL), the brainstem (including the dorsal raphe nucleus and parabrachial nucleus), other brain regions (such as the dorsal striatum, infralimbic cortex, nucleus accumbens and cerebellum) as well as peripheral targets (such as the gastrointestinal system). In this Article, we have delineated a brain circuit (IGLNPY–SCN) that is critical for driving food-anticipatory activity in adult mice. The functional assembly of this circuit requires innervation by retinal ipRGCs to the SCN during a critical window. The proposed model suggests crosstalk between an FEO (or FEOs) and the IGL, in which IGL neurons act as a node of connection between the FEOs and the central pacemaker in the SCN. Under TRF, inhibitory signals from IGLNPY neurons modulate the SCN function, causing reduced firing activity, and therefore allowing signals from the FEO (or FEOs) to drive robust food-anticipatory activity. The IGL could also be part of the FEO (or FEOs), as previously suggested7. IGLNPY neurons send projections to several brain regions and, therefore, a role of any of these non-SCN projections in modulating food-anticipatory activity should not be excluded. How feeding-related stimuli modulate the FEO (or FEOs) and possibly the IGL are unknown. Different humoral signals, such as ghrelin and insulin, are strong candidates for modulating the FEO (or FEOs) and IGL.
Supplementary information
Supplementary Information
This file contains supplementary discussion and references
Supplementary Video 1 | SCN NPY levels from an adult control mouse
Three independent experiments were performed with similar results. Maximum projection of 30 z-planes; slice spacing: 0.8 μm. 593.35 x 296.68 mic (2048 x 1024); 16-bit.
Supplementary Video 2 | SCN NPY levels from an adult Opn4DTA/DTA mouse
Three independent experiments were performed with similar results. Maximum projection of 30 zplanes; slice spacing: 0.8 μm. 593.35 x 296.68 mic (2048 x 1024); 16-bit.
Supplementary Video 3 | SCN NPY levels from an adult WT mouse
Three independent experiments were performed with similar results. Maximum projection of 30 z-planes; slice spacing: 0.8 μm. 593.35 x 296.68 mic (2048 x 1024); 16-bit.
Supplementary Video 4 | SCN NPY levels from an adult P0 enucleated WT mouse
Three independent experiments were performed with similar results. Maximum projection of 30 zplanes; slice spacing: 0.8 μm. 593.35 x 296.68 mic (2048 x 1024); 16-bit.
Supplementary Video 5 | SCN NPY levels from an adult P40 enucleated WT mouse
Three independent experiments were performed with similar results. Maximum projection of 30 zplanes; slice spacing: 0.8 μm. 593.35 x 296.68 mic (2048 x 1024); 16-bit.
Supplementary Video 6 | SCN NPY levels from an adult P90 enucleated WT mouse
Three independent experiments were performed with similar results. Maximum projection of 30 zplanes; slice spacing: 0.8 μm. 593.35 x 296.68 mic (2048 x 1024); 16-bit.
Rights and permissions
About this article
Cite this article
Fernandez, D.C., Komal, R., Langel, J. et al. Retinal innervation tunes circuits that drive nonphotic entrainment to food. Nature 581, 194–198 (2020). https://doi.org/10.1038/s41586-020-2204-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2204-1
This article is cited by
-
A Comprehensive Overview of the Neural Mechanisms of Light Therapy
Neuroscience Bulletin (2024)
-
Melanopsin-mediated optical entrainment regulates circadian rhythms in vertebrates
Communications Biology (2023)
-
Circadian disruption: from mouse models to molecular mechanisms and cancer therapeutic targets
Cancer and Metastasis Reviews (2023)
-
Day time-restricted feeding shows differential synchronizing effects on age-related changes of serotonin metabolism in SCN and the pineal gland in male Wistar rats
Biogerontology (2022)
-
Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions
Cellular and Molecular Life Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.