
Abstract
Aims: Although transcatheter aortic valve implantation (TAVI) is officially indicated for severe aortic stenosis (AS) patients at intermediate or higher surgical risk, the procedure is now increasingly being performed in patients who are at low surgical risk. Data on the benefit of TAVI in this patient population are limited. We therefore aimed to perform an updated meta-analysis of all published randomised controlled trials (RCTs) and propensity score-matched studies comparing TAVI versus surgical aortic valve replacement (SAVR) in patients at low surgical risk.
Methods and results: We conducted a systematic review and meta-analysis of RCTs and observational studies with propensity score matching (PSM) of TAVI versus SAVR in patients who are at low surgical risk (mean STS score <4% and/or logistic EuroSCORE <10%). The primary outcome was mortality (examined at 30 days, one year and the longest available follow-up). The secondary outcomes included procedural complications. Nine studies (n=6,124) were included. TAVI was associated with a numerically, but not statistically, significant reduced mortality at 30 days (1.45% vs 2.1%, p=0.05), and similar mortality at one year (5.1% vs 5.0%, p=0.74) and a median of two years (10.8% vs 9.8%, p=0.15). For both time points, there was significant heterogeneity between RCT/PSM studies, with the former suggesting survival advantage for TAVI and the latter for SAVR. In terms of periprocedural complications, TAVI was associated with reduced risk for stroke, bleeding and renal failure and an increase in vascular complications and pacemaker implantation.
Conclusions: In patients who are at low surgical risk, TAVI seems to be associated with equivalent mortality up to a median follow-up of two years compared to SAVR. More data are required before TAVI can be routinely considered as an alternative to SAVR in low-risk patients.
Introduction
The availability of transcatheter aortic valve implantation (TAVI) has revolutionised the treatment of patients with symptomatic aortic stenosis (AS) over the past decade.
TAVI was shown to be superior to conservative management in patients who are inoperable1, and at least equivalent to surgical aortic valve replacement (SAVR) among patients who are at high2,3 and intermediate4,5 surgical risk.
The annual volume of TAVI procedures is growing exponentially. Real-world data show a decline in the average risk profile of TAVI patients6. The next step in the evolution of TAVI is the expansion of its indication to include low-risk patients as well. Data on the outcomes of TAVI versus SAVR in low-risk patients are limited: a meta-analysis published in 2018 found that in low-risk patients TAVI was associated with an increase in intermediate-term mortality7, but this was based on data from two small randomised controlled trials (RCTs) and four propensity score-matched (PSM) studies only. The recent publication of two pivotal RCTs of TAVI versus SAVR in low-risk patients8,9 offered an opportunity to re-examine the available data on the balance between TAVI/SAVR in this patient population. A subsequent updated meta-analysis of RCTs comparing TAVI versus SAVR in all risk groups found a survival advantage for TAVI which was consistent across all risk strata10. Although RCTs constitute the highest level of clinical evidence, their selection process entails an inherent bias11 and therefore caution should be employed when applying their conclusions to real-world patients, who in many cases would not fulfil the inclusion criteria of RCTs. In this setting, it is important to examine whether the results of real-world patients are consistent with those reported in RCTs.
We therefore performed an updated meta-analysis of all published RCTs and PSM studies comparing TAVI versus SAVR in patients at low surgical risk.
Materials and methods
The registered study protocol is available on PROSPERO (CRD42017060014). We searched Medline, Embase, and Cochrane CENTRAL from April 2017 (the latest search in our previous meta-analysis) up to June 2019, for studies comparing TAVI and SAVR.
Studies that met the following criteria were considered for inclusion:
– Study design was either an RCT or observational study using propensity score matching to create patient groups with similar baseline characteristics.
– Reported the mean surgical risk of the TAVI and SAVR groups using the Society of Thoracic Surgeons (STS) score and/or logistic EuroSCORE.
– Patients were at low surgical risk for SAVR, as defined by a mean STS score <4% and/or logistic EuroSCORE <10%. In case the authors reported both STS and logistic EuroSCORE data and the results were discordant, we used the STS data for eligibility.
All titles and abstracts were screened, and those thought possibly to meet the inclusion criteria were screened for eligibility using the full text.
Studies were excluded if:
– The manuscript did not include data on overall mortality for at least short-term follow-up (either in-hospital or 30 days).
Two reviewers (G. Witberg and U. Landes) independently extracted the data; conflicts were resolved by a third reviewer (A. Lador). For all outcomes, data were extracted for the largest patient population evaluated. The primary outcomes were all-cause mortality (30-day, one-year, and the longest available follow-up). Secondary outcomes were periprocedural (in-hospital/30-day) outcomes: cerebrovascular accident (CVA), myocardial infarction (MI), acute kidney injury (AKI), bleeding (as defined in the individual studies – 5/8 studies used the Valve Academic Research Consortium [VARC]/VARC-2 criteria12,13), vascular complications, and the need for pacemaker implantation (PMI).
Two authors assessed the risk of bias (G. Witberg and U. Landes). Cochrane’s handbook tool14 was used to assess the RCTs. The Newcastle-Ottawa scale was used to assess the quality of the PSM studies15. The reviewers resolved conflicts through consensus.
A systematic review and meta-analysis was performed in compliance with the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement14. Meta-analysis was performed using Review Manager (RevMan) software. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Heterogeneity between the included trials was assessed using the chi-squared test for heterogeneity and the I2 measure of inconsistency16, but the choice between a random/fixed effects model was not determined by the results of statistical tests for heterogeneity, but rather, as recently recommended by a scientific statement of the American Heart Association17, by evaluating the functional similarity between the included studies and the goal of estimating a common effect size that will be applicable to similar populations to those included in this meta-analysis. Fixed effects, pooled estimates of odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using the Mantel-Haenszel method. For random effects, the DerSimonian and Laird random method was used. Reported values are two-tailed, and hypothesis-testing results were considered significant at p<0.05.
Comparisons were subcategorised by the study’s design (RCT/PSM).
Results
The results of the study selection process are shown in Figure 1. Our initial search yielded 1,827 citations, 58 of which were judged to be potentially eligible and underwent full text review. Ten studies were found to be eligible for inclusion after full text review, four RCTs8,9,18,19 and six PSM studies20,21,22,23,24,25. There were two studies from the OBSERVANT registry20,23, of which only the study by Rosato et al23 intentionally included only low surgical risk patients, while the study by Fraccaro et al20 was limited to patients older than 80 and intended to include also intermediate-risk patients. We therefore included the study by Rosato et al and excluded the study by Fraccaro et al as a duplicate publication. From the study by Piazza et al22, we used the mortality data on the subgroup of patients with STS score <4 rather than the overall results that included intermediate-risk patients as well.
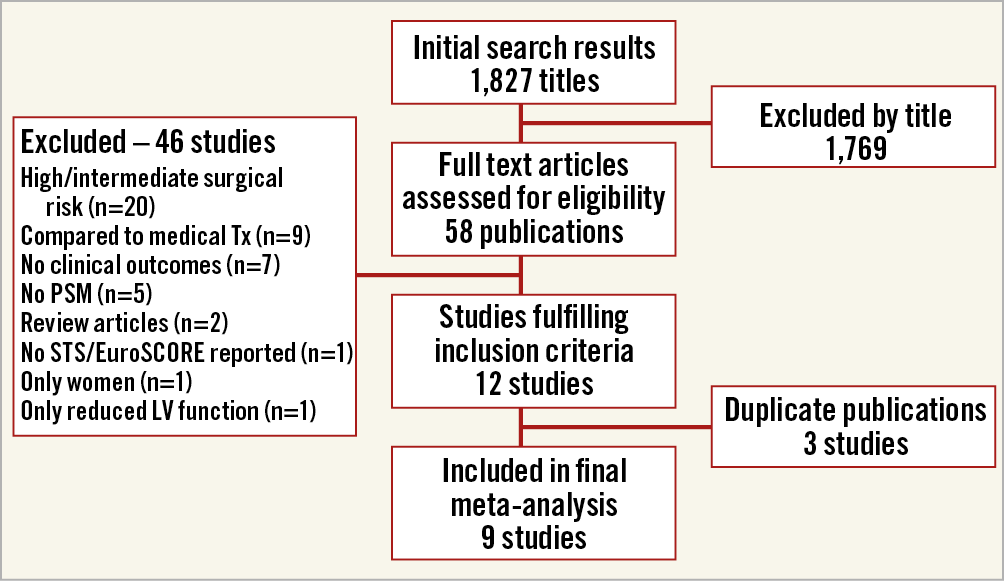
Figure 1. Study selection process for inclusion in the meta-analysis. LV: left ventricle; PSM: propensity score matching; SAVR: surgical aortic valve replacement; STS: Society of Thoracic Surgeons
The aggregated sample size was 6,124 patients (2,764 RCT and 3,360 PSM patients). The characteristics of the trials included in this meta-analysis are shown in Supplementary Table 1.
All of the PSM studies were ranked as good quality according to the Newcastle-Ottawa scale (score range 7-9). In addition, all of the RCTs were at low risk of bias in terms of the generation of randomisation and concealment of allocation. Due to the invasive nature of the examined interventions, none was blinded – Supplementary Figure 1 contains the full assessment of bias.
A summary of the major comorbidities, and of the clinical and echocardiographic characteristics of the patients included in the meta-analysis is presented in Supplementary Table 2.
CLINICAL OUTCOMES
Forest plots for overall mortality are shown in Figure 2. Peri-procedural mortality showed a numerically, but not statistically significant reduced mortality with TAVI vs SAVR (1.4% [44/3,086] and 2.1% [64/3,038], respectively [OR 0.68, 95% CI: 0.46-1.00, p=0.05]). One-year mortality was 5.1% (109/2,125) and 5.0% (102/2,028) for TAVI and SAVR, respectively (OR 1.05, 95% CI: 0.79-1.39, p=0.74). At the longest available follow-up (median two years), the risk of mortality was 10.8% (264/2,432) and 9.8% 229/2,333) for TAVI and SAVR, respectively (OR 1.15, 95% CI: 0.95-1.40, p=0.15).
For both one-year and the longest available mortality, there was significant heterogeneity between the PSM/RCT subgroups (I2=82.5% and 75.3%), with the RCT group suggesting a trend towards reduced mortality with TAVI at one year (OR 0.65, 95% CI: 0.40-1.05, p=0.08, I2=0%), or no difference for a median of two years (OR 0.86, 95% CI: 0.62-1.22, p=0.40), and the PSM group suggesting a trend towards increased mortality with TAVI at one year (OR 1.35, 95% CI: 0.95-1.91, p=0.09, I2=0%), and significant increase in mortality at a median of two years (OR 1.32, 95% CI: 1.05-1.67, p=0.02) (Figure 2A-Figure 2C).
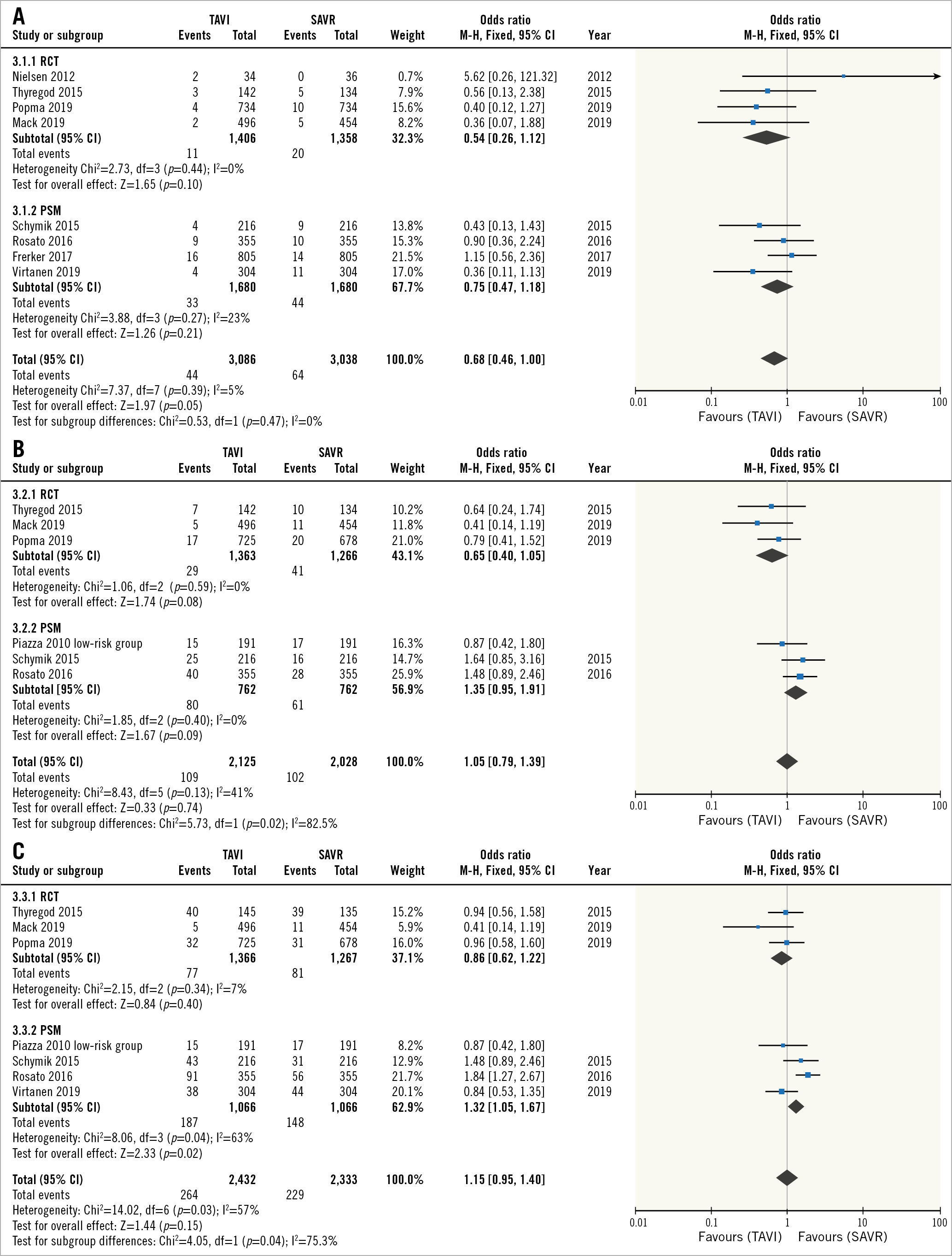
Figure 2. Mortality. Forest plots of the odds ratios for 30-day (A) and one-year (B) and longest available (median two-year) (C) mortality for TAVI versus SAVR. SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
PERIPROCEDURAL COMPLICATIONS
Periprocedural complications are summarised in Supplementary Table 3 and the corresponding forest plots are shown in Figure 3. TAVI was associated with reduced risk for CVA, AKI and bleeding (Figure 3A, Figure 3C, Figure 3D), There was no difference in the risk for MI between TAVI and SAVR (Figure 3B), and the risk for PMI and vascular complications was higher with TAVI (Figure 3E, Figure 3F).
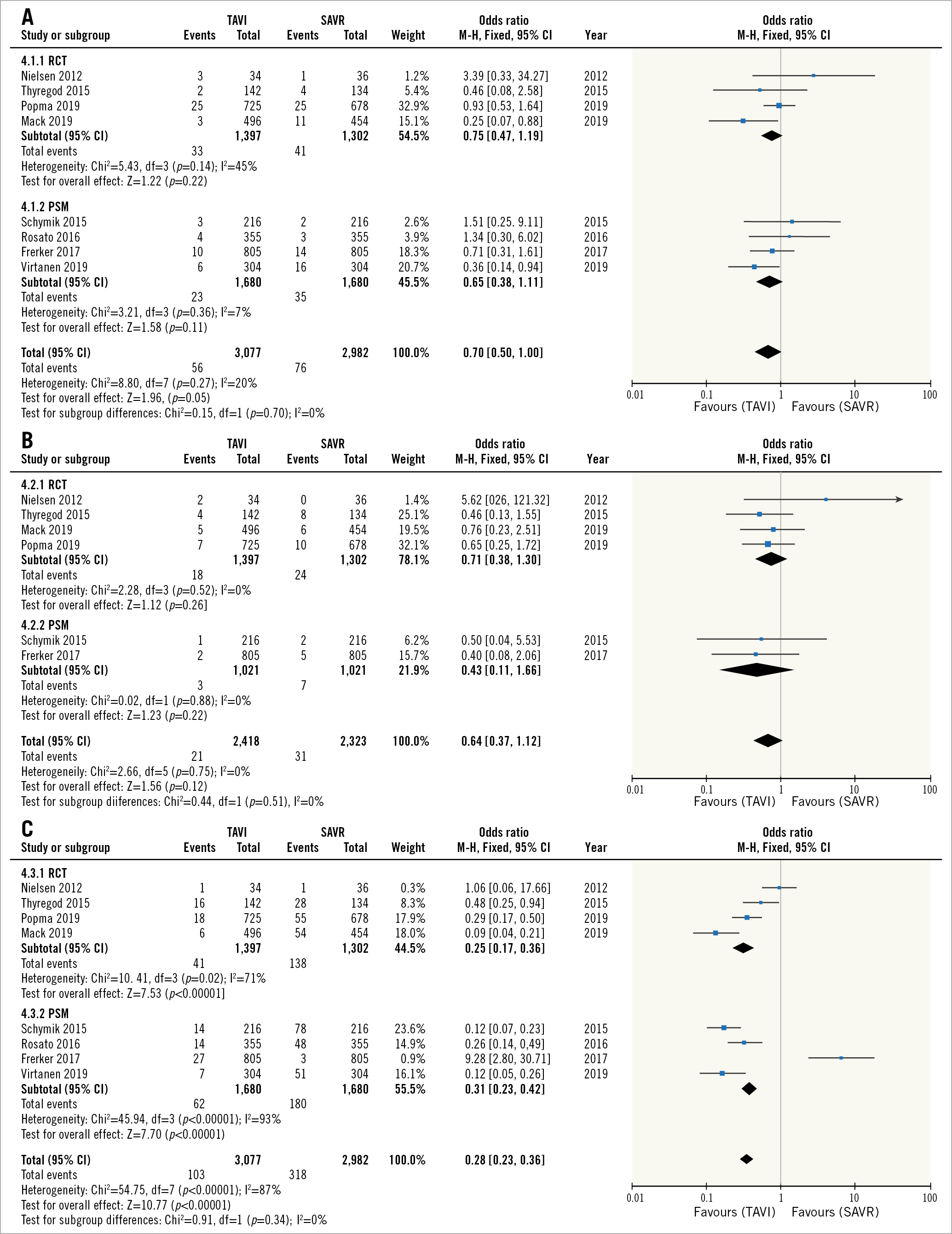
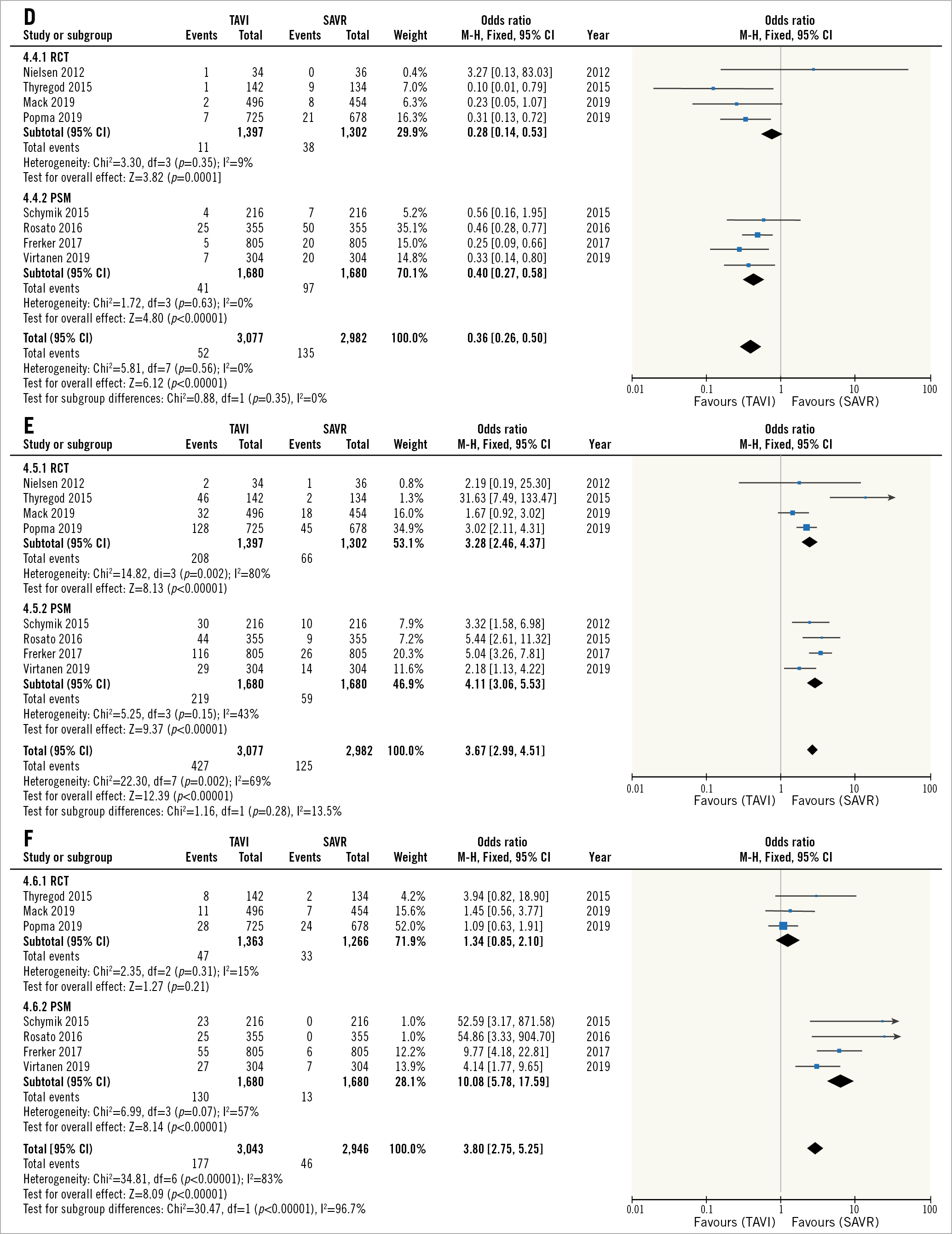
Figure 3. Periprocedural complications. Forest plots of the odds ratio for periprocedural CVA (A), MI (B), major bleeding (C), AKI (D), PMI (E), and vascular complications (F) after TAVI versus SAVR in low-risk patients. AKI: acute kidney injury; CVA: cerebrovascular accident; MI: myocardial infarction; PMI: pacemaker implantation; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
Increased risk for vascular complications with TAVI was much lower in the RCTs compared to PSM studies (OR 1.34 and OR 10.01, respectively), and in fact was not statistically different between TAVI versus SAVR in the RCT patients only (p=0.21).
A trial sequential analysis for mortality at a median follow-up of two years suggested that a sample size of 15,463 patients will be required for a definitive meta-analysis (compared to 4,675 patients included in this meta-analysis for this outcome) and that the current Z-score did not cross either the futility or O’Brien-Fleming boundaries (Figure 4).
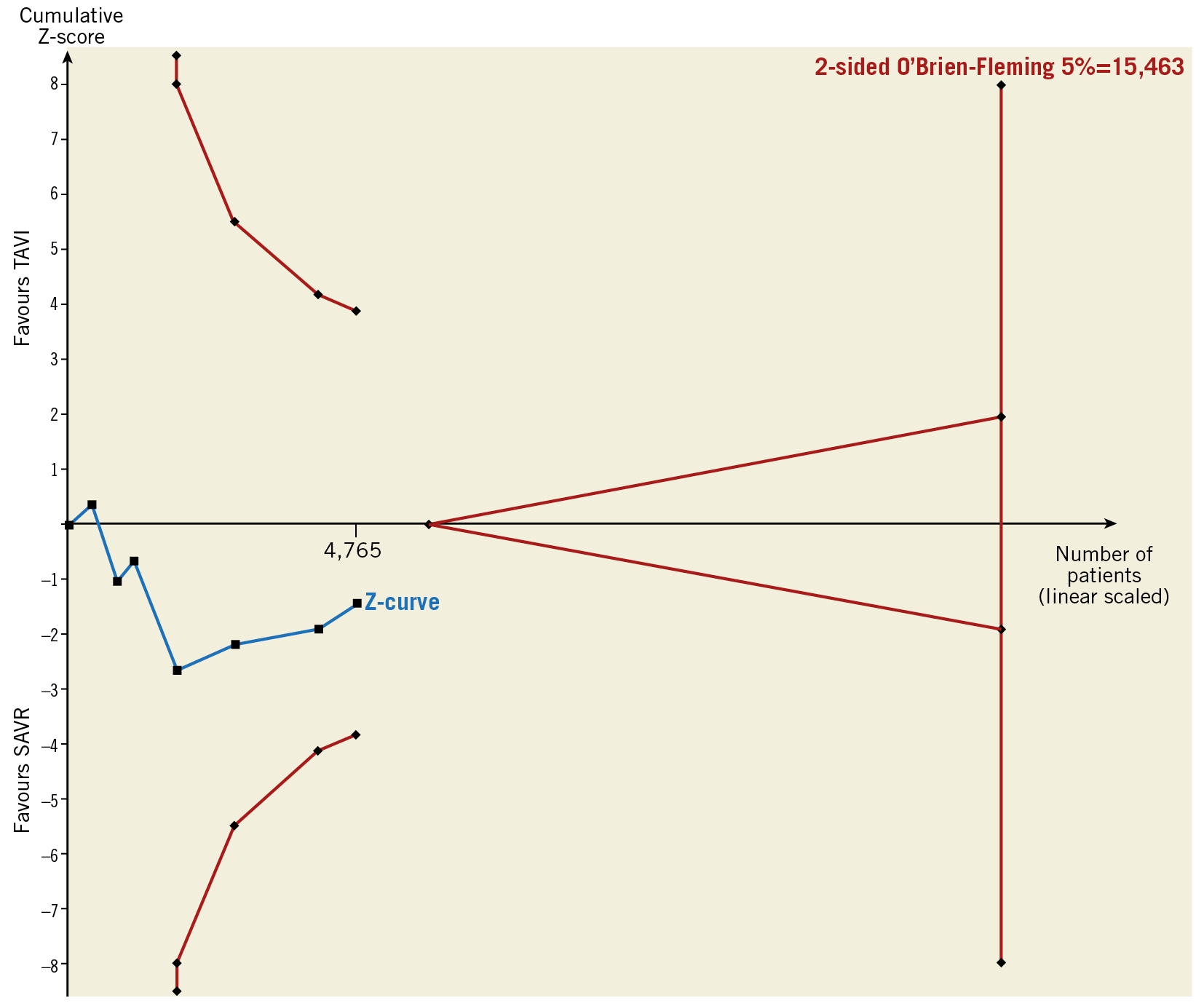
Figure 4. Trial sequential analysis for longest available (median two years) mortality.
Discussion
We conducted a systematic review and meta-analysis of all RCTs and PSM studies evaluating TAVI versus SAVR for patients with severe AS who are at low surgical risk.
Our main findings are:
– Mortality for a median of two years was low, as would be expected in low-risk patients.
– TAVI was associated with lower short-term mortality (32% reduction) and CVA (30% reduction), although both were statistically non-significant (p=0.05 for both), and higher risk for vascular complications and PMI.
– Mortality at one year and a median of two years of follow-up was similar between TAVI and SAVR, but there was significant heterogeneity between study types with RCTs suggesting better results for TAVI at one year and equivalent results at two years, respectively, while PSM studies suggested better results for SAVR at both one year and a median of two years.
– Our trial sequential analysis suggests that more data are needed in order to draw definitive conclusions regarding the differences in mortality between TAVI and SAVR in low surgical risk patients.
Throughout the rapid expansion of TAVI volumes over the recent decade, the surgical risk profile of real-world patients has been lower than that of those included in the randomised trials on whom the formal practice guideline recommendations are based. For example, a report from a national registry in Germany recently showed that, between 2012 and 2014, 85% of severe AS patients who were at intermediate surgical risk were treated by TAVI26. Likewise, the median STS score of patients undergoing TAVI in the USA between 2012 and 2015 was in the intermediate-risk category (6.5)6, while the first large-scale RCT of TAVI versus SAVR in intermediate-risk patients was only published in 20164, and the first reference to TAVI as an alternative to SAVR in patients who are not at high surgical risk in the ESC guidelines was only published during 201727. The same is true for low surgical risk patients, with registry reports on this patient population dating back to 201524, while the first large-scale RCTs were only published in 20198,9, and current guidelines do not yet consider TAVI suitable for low surgical risk patients. In such settings, a meta-analysis is important, as it provides physicians with the most comprehensive data regarding the balance between TAVI and SAVR and can include data from both RCTs and real-world patients. The last point is significant since, although RCTs are considered the best source of scientific data to guide clinical practice, they carry an inherent selection bias11. Therefore, it is important to see whether their results are consistent with those of real-world patients and, if that is not the case, consider the reasons for this discrepancy.
The current meta-analysis represents a significant improvement in the quality of data compared to our previous publication on the same subject7. First, the sample size is much larger (6,124 compared to 3,484 patients) and, more importantly, the percentage of patients enrolled in RCTs is much more significant (45%).
When we compare the results regarding mortality, the periprocedural mortality is lower than previously reported (1.4% and 2.1% compared to 2.2% and 2.6% for TAVI and SAVR, respectively), with a 32% reduction in mortality with TAVI at 30 days. For one-year mortality and beyond (up to a median of two years), there seems to be a discrepancy between the RCT and PSM studies. It seemed that results in the RCTs favoured TAVI while those of the PSM studies favoured SAVR (Figure 2B, Figure 2C). The most likely explanation for these results is the difference between the PSM and RCT studies regarding the era in TAVI evolution they represent: while practically all of the patients included in the RCT group were treated during 2016-2017, the PSM studies included patients who underwent TAVI during 2008-2012. During this time gap, much progress was made in all aspects of TAVI. First and foremost, newer-generation TAVI devices have become the standard of care and replaced first-generation devices. However, numerous other aspects regarding TAVI have changed as well: more experience has been gained in the assessment, triage, preparation and periprocedural management of patients, as well as in performing the TAVI procedures. The use of cardiac computed tomography (CT) for preprocedural planning has become the standard of care, delivery sheath sizes have been reduced leading to a decrease in the use of non-femoral access, conscious sedation has become the standard of care rather than general anaesthesia, and clinical pathways for earlier mobilisation and discharge have been developed and implemented. This explanation is also supported by some of the results regarding periprocedural complications. Looking at vascular complications (Figure 3F), although overall the risk for vascular complications is higher in TAVI compared to SAVR, this was driven solely by the PSM studies, while in the RCTs (whose participants were treated using smaller sheaths and were all assessed by CT to assess the optimal access), the risk for vascular complications was not statistically significant between TAVI and SAVR. Looking at the risk for PMI (Figure 3E), although in both groups the risk was much greater with TAVI, the odds ratio was 21% lower in the RCTs compared to PSM studies. Again, this is probably related to the better understanding of the pathophysiologic mechanisms of conduction abnormalities post TAVI and possible strategies to mitigate them that accumulated with more clinical and research experience concerning this issue28.
If we examine the data on periprocedural outcomes, it seems that the less invasive nature and shorter admission associated with TAVI may be of great benefit for low-risk patients (significant reductions in mortality, CVA, AKI and bleeding). In the current analysis, these benefits did not translate into mortality benefits at longer follow-ups, but it should be noted that the one-year and beyond mortality analyses were dominated by PSM studies, so longer follow-up data from the large RCTs8,9 are required to examine whether these short-term benefits eventually translate into long-term benefits as well.
These results, although only hypothesis-generating, can be viewed as a tell-tale sign that the iterations in TAVI devices as well as more experience in the care of TAVI patients accumulated over the past decade do result in improved outcomes for TAVI patients compared to the past. Whether this improvement will eventually sway the balance between TAVI and SAVR towards the transcatheter approach in patients at low surgical risk still requires more evidence.
Limitations
Our study has several limitations. First, we did not have access to individual patient data and could not present subgroup analysis according to access site or device type for the PSM studies, which may have different risk/benefit profiles (subgroup analysis for the RCTs has been presented by Siontis et al10). Second, the currently accepted risk scores for TAVI are actually based on historical SAVR patients and may not be able to describe the risks involved with TAVI accurately, causing misclassification of patient risk. Third, it should also be remembered that the latest two low-risk RCTs8,9 included only patients who were very good candidates for TAVI (femoral access, high coronaries, no severe valve calcifications, etc.) and thus the results may not reflect the balance between TAVI and SAVR in real-world low surgical risk patients appropriately. Fourth, the follow-up period in the studies included in this meta-analysis (median two years) is insufficient to assess long-term differences between TAVI and SAVR, especially in low-risk patients whose life expectancy post TAVI/SAVR is expected to be longer than the average TAVI patient in current clinical practice. Fifth, three of the studies did not use the VARC/VARC-2 definitions for the secondary outcomes. Finally, some additional issues that are beyond the scope of this meta-analysis need to be taken into account when choosing between TAVI and SAVR. Long-term prosthetic valve durability, an issue on which data are still limited29, is of vital importance for low-risk patients. The cost-effectiveness of TAVI as opposed to SAVR will also need to undergo extensive scrutiny before expanding TAVI indications to include low-risk patients – a change that will have wide-scale economic implications in countries with predominantly public healthcare systems.
Conclusions
The currently available data suggest that for low-risk patients TAVI is associated with a trend towards improved periprocedural mortality and CVA compared to SAVR, and is equivalent to SAVR in terms of mortality for up to a median follow-up of two years. The current evidence base is still insufficient to derive definitive conclusions. Longer follow-up data from the published low-risk TAVI RCTs and data from those still underway (the NOTION 2 trial - NCT02825134) are required before TAVI can be routinely considered an alternative for SAVR in low-risk severe AS patients.
|
Impact on daily practice Our results suggest that TAVI should not be a priori denied to patients who are at low surgical risk for SAVR, something which should be reflected by the major cardiology practice guidelines. Until more definitive data on the balance between TAVI and SAVR in this patient population are available, Heart Team discussions concerning the optimal management of low-risk patients with severe AS must consider the remaining limitations of TAVI and make judicious, evidence-based decisions for individual patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.




