
Abstract
Aims: The VIVALL study aims to investigate the technical feasibility, safety and performance of the ALLEGRA transcatheter heart valve (THV) for the treatment of failing surgical aortic valves (SAV).
Methods and results: Thirty patients with failing SAV were investigated. An independent combined Data Safety Monitoring-Clinical Events Committee (DSM-CEC) and core lab adjudicated adverse events, patient safety and echocardiograms, respectively. Primary endpoints were invasive post-procedure mean pressure gradient (performance) and 30-day survival (safety). Of the treated patients (78.6±6.0 years, 50% female, STS score 4.5±2.1% and EuroSCORE II 9.2±4.3%), the majority (90%) had a small SAV (true inner diameter ≤22 mm). Implantation was successful in all but one patient (96.7%). Overall, the invasively assessed preoperative mean pressure gradient was significantly reduced from 37.1±13.3 mmHg to 11.6±3.7 mmHg. At 30 days, all-cause mortality and new pacemaker implantation were both 0% and the effective orifice area increased from 1.18±0.58 cm2 at baseline to 1.4±0.52 cm2. Paravalvular regurgitation was “none or trace” in 100% of the cases.
Conclusions: Transfemoral implantation of the ALLEGRA THV is feasible and safe in patients with failing SAV. Haemodynamic outcomes and a 100% survival rate after 30 days suggest that the ALLEGRA THV might be a valid option for valve-in-valve treatment.
Introduction
Worldwide, more than 200,000 surgical aortic valve replacements (SAVR) are performed annually. Typically, affected patients are 65 years and older. Due to a lower risk of bleeding and thrombotic events and the desire to reduce anticoagulants, more and more bioprostheses are being used rather than mechanical valves1.
Nevertheless, these bioprosthetic valves fail due to the degenerative processes within 10 to 20 years2,3. Structural valve deterioration can result from leaflet degeneration and failure, as evidenced by valve stenosis, regurgitation, or a combination of both4. In the past, repeat surgical valve replacement has been the standard treatment for these patients. However, this patient population may often be at high risk for further surgery due to their advanced age and additional comorbidities. Also, “redo” cardiac surgery is associated with an increased morbidity and mortality risk4,5,6.
Previously, this unmet clinical need led to off-label use implantations of various transcatheter valve technologies for the treatment of degenerated bioprosthetic surgical heart valves to avoid redo open heart surgery5,7,8,9,10,11.
Recently, numerous reports have suggested that minimally invasive transcatheter aortic valve-in-valve implantation (ViV) is a potential treatment option for patients with failing surgical bioprostheses at elevated surgical risk12,13,14,15,16.
The first-in-human clinical studies with the ALLEGRA transcatheter heart valve (THV) (New Valve Technology GmbH, Hechingen, Germany) showed favourable results, with a haemodynamic performance comparable to other CE-approved THVs17,18. Lately, the ViV function of the ALLEGRA was evaluated in vitro (hydrodynamic testing) as well as in a small series of ViV-TAVI (n=4), showing good hydrodynamic and haemodynamic outcomes19,20. Due to these favourable properties indicating a potential role for this novel THV in ViV-TAVI, the VIVALL study (ClinicalTrials.gov: NCT03287856) was initiated to evaluate the clinical performance of the ALLEGRA for the ViV indication.
Methods
STUDY DESIGN
The VIVALL study is a prospective, multicentre, single-arm study with defined follow-ups after 30 days, 6 and 12 months. The objective of the study is to investigate the technical feasibility of implanting the ALLEGRA THV into failing surgical bioprosthetic aortic valves and to describe the safety and performance profiles. The study complies with the Declaration of Helsinki and was approved by all involved ethics committees and the local competent authority. Patients were informed about the study details and provided written informed consent prior to study participation. All data were adjudicated by an independent combined Data Safety Monitoring-Clinical Events Committee (DSM-CEC) and a core lab with regard to adverse events, patient safety and echocardiograms, respectively. All eligibility criteria are shown in Supplementary Table 1.
STUDY POPULATION
Thirty symptomatic patients with a failing surgical aortic bioprosthesis and increased surgical risk for a “redo” operation (as assessed by the Heart Team) and candidates for a transcatheter aortic valve implantation (TAVI) were enrolled at five German sites: the University Heart Center Hamburg (n=22), the Heart Center Brandenburg in Bernau & Brandenburg Medical School (n=4), Segeberger Clinic, Heart Center (n=2), Asklepios Clinic St. Georg (n=1) and the University Heart Surgery Clinic Halle (Saale) (n=1).
DEVICE AND VALVE IMPLANTATION
All patients underwent TAVI into failing surgical bioprostheses with the ALLEGRA THV. The transfemoral ALLEGRA TAVI system consists of the ALLEGRA THV with a trileaflet bovine pericardial tissue valve and a self-expanding nitinol stent and the ALLEGRA Delivery System TF. The main features of the ALLEGRA TAVI System TF are (1) radiopaque markers on both the ALLEGRA delivery system and the stent, which facilitate accurate placement during the procedure, (2) the Permaflow technology that allows positioning and implantation without flow obstruction and the need for rapid pacing, and (3) the ALLEGRA THV with a trileaflet bovine pericardial tissue valve.
STUDY ENDPOINTS AND ASSESSMENTS
Patients are assessed at baseline, procedure, discharge, 30 days, 6 and 12 months. The primary performance endpoint is postoperative invasive mean pressure gradient (expected to be <20.8 mmHg); the primary safety endpoint is 30-day survival (expected to be >72.7%), both assessed in the intention-to-treat (ITT) population.
Secondary endpoints include technical implantation success, assessments of New York Heart Association (NYHA) class and the necessity for new pacemaker implantations. Additional endpoints, including cardiovascular mortality, haemodynamic parameters and early safety, were adjudicated according to the VARC-2 guidelines21. An independent core lab (coreLab Black Forest GmbH, Bad Krozingen, Germany) evaluated all echocardiographic assessments (Supplementary Table 2).
STATISTICAL ANALYSIS
All recorded variables were analysed on a per visit basis and compared with baseline values using appropriate descriptive summary statistics (continuous and ranked data: sample size, mean, standard deviation, minimum, first quartile, median, third quartile, maximum; categorical data: sample size, absolute and relative frequency).
The descriptive statistics of a variable were calculated for each visit and for each defined change. Changes were calculated as differences for each visit relative to screening/pre-treatment value only.
For the success of the study, two null hypotheses had to be rejected. The primary performance endpoint - invasively measured postoperative mean pressure gradient - was evaluated for pooled data as described above, except that postoperative data of eligible patients without a successful ALLEGRA THV implantation were substituted with the preoperative values. We used a one-sample t-test with an H0 ≥20.8 mmHg mean pressure gradient. The H0 of the primary safety endpoint was “30-day survival” >72.8% and tested by the lower one-sided Clopper-Pearson confidence interval, corresponding to a lower one-sided chi-square (χ2) test. The critical alpha level was one-sided 5%. Both tests had to be successful; therefore, adjustment for multiple testing was necessary. The analysis was pre-specified in the clinical investigation plan (CIP) and the statistical analysis plan (SAP). All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
PATIENTS
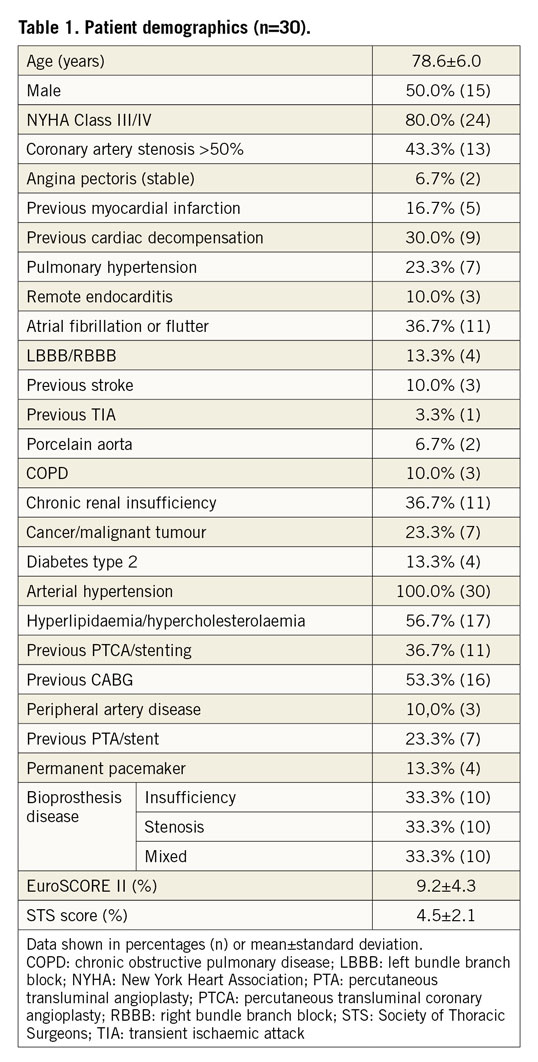
Between August 2017 and September 2018, 30 patients were treated with the transfemoral ALLEGRA THV. Patient demographics and baseline data are shown in Table 1. Mean age was 78.6±6.0 years, 50% were female and 80% were severely symptomatic in NYHA Class III or IV. EuroSCORE II and STS score were 9.2±4.3% and 4.5±2.1%, respectively. Coronary artery disease (CAD, with stenosis >50% and/or previous myocardial infarction) was present in 53.3% of patients. Also, 53.3% had atrial fibrillation or atrial flutter and conduction disturbances (left bundle branch block [LBBB]/right bundle branch block [RBBB] and atrioventricular [AV] block), and 100% of the patients suffered from arterial hypertension.
Surgical bioprosthesis dysfunction modes included stenosis (33.3%), insufficiency (33.3%), and combined stenosis and insufficiency (33.3%). The majority of the failing surgical valves were Mitroflow (Sorin Group USA Inc., Arvada, CO, USA) (43.3%), and 80.0% of the surgical bioprostheses had a true inner diameter of ≤21 mm. The characteristics of the surgical valves are summarised in Supplementary Table 3.
PROCEDURAL RESULTS
The ALLEGRA THV was successfully implanted in 96.6% (29) of the patients. In one case, the ALLEGRA THV migrated into the aorta, resulting in severe paravalvular regurgitation and haemodynamic instability requiring subsequent implantation of an Evolut™ R valve (Medtronic, Minneapolis, MN, USA). In three patients (10%), the ALLEGRA THV was retrieved, reloaded and successfully implanted. In 90% (27) of the study population, a 23 mm ALLEGRA THV was used and in 10% (3) a 27 mm THV. Predilatation and post-dilatation were performed in 6.7% and 56.7% of the cases, respectively (Table 2). The invasively measured preprocedural mean pressure gradient was reduced from 37.1±13.3 mmHg to 11.6±3.7 mmHg post procedure in the per-protocol analysis (Figure 1A). For the conservative analysis of the primary performance endpoint, the postoperative value of one patient without a successful ALLEGRA THV implantation was substituted with the preoperative value, leading to an invasively assessed post-procedural mean pressure gradient of 12.4±5.8 mmHg. Eight patients showed a severe patient-prosthesis mismatch (PPM) (indexed effective orifice area [EOAi] <0.65 cm2/m2) at discharge, resulting in an overall device success rate of 60% (18). Nevertheless, subgroup analysis according to the multislice computed tomography (MSCT) perimeter-derived inner diameter (ID: >22 mm, >21 mm, <21 mm-≥20 mm, <20 mm-≥19 mm, <19 mm) (Supplementary Figure 1A) showed similar acute invasive haemodynamic improvements, especially in the very small bioprostheses (Supplementary Figure 1B), as well as acceptable echocardiographic haemodynamics at discharge and after 30 days (Supplementary Figure 2A-Supplementary Figure 2C).
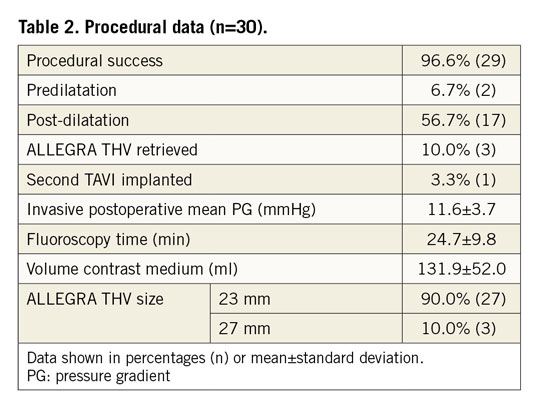
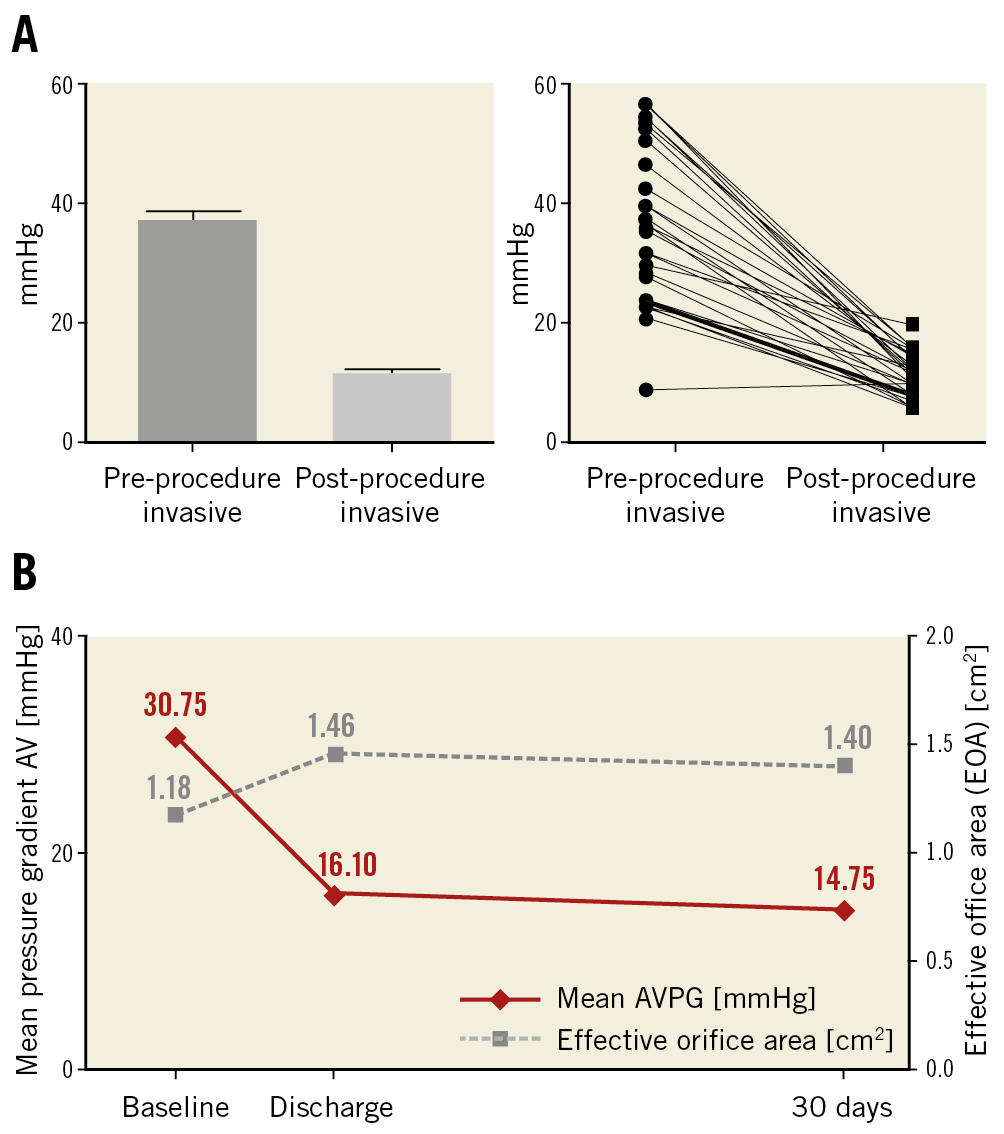
Figure 1. Valve haemodynamics. A) Invasive mean gradient before and after ALLEGRA THV implantation. B) Echocardiographic mean aortic gradient across the bioprostheses and mean effective orifice area (EOA) at baseline, discharge and 30 days.
THIRTY-DAY OUTCOMES
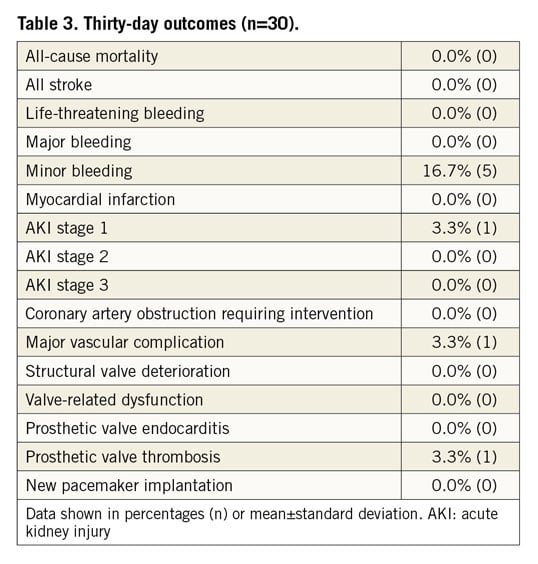
Twenty-eight patients underwent the 30-day follow-up. One patient missed the visit and the patient who received the second TAVI was followed up via a safety follow-up, assessing only serious adverse events. Early safety was achieved in 93.3% (28) of the patients. The thirty-day data are outlined in Table 3. The survival rate was 100% (primary safety endpoint) and there was no stroke, no life-threatening or major bleeding and no myocardial infarction. More than half of the patients (56%) were treated with cerebral embolic protection devices (Sentinel® Cerebral Protection System; Claret Medical, Inc., Santa Rosa, CA, USA) during the ViV implantation. Minor bleedings were observed in 16.7% (5) and major vascular complications in 3.3% (1) with one dissection of the iliac artery during the procedure. Acute kidney injury (AKI) stage 1 was observed in 3.3% (1) of the patients.
Echocardiographic measurements demonstrated a consistent decrease of the mean gradient across the aortic valve from 30.6±12.6 mmHg at baseline to 16.1±6.4 mmHg and 14.8±6.5 mmHg at discharge and after 30 days, respectively, whereas the effective orifice area (EOA) increased to 1.46±0.53 cm2 and 1.40±0.52 cm2 at discharge and 30 days, respectively, compared to 1.18±0.58 cm2 at baseline (Figure 1B). Likewise, mean EOAi improved from baseline to discharge and 30 days from 0.64±0.31 cm2/m2 to 0.78±0.25 and 0.75±0.25 cm2/m2, respectively.
There was one case (3.3%) with evidence of subclinical prosthetic valve thrombosis after 23 days under treatment with rivaroxaban, which was detected during the standard echo examination at the 30-day follow-up visit. The patient was without symptoms, and mean pressure gradients at discharge and 30 days (19 mmHg and 15 mmHg, respectively) were without clear pathological findings when compared with 30 mmHg at baseline. The patient was readmitted to hospital and discharged four days later without symptoms after a change to oral anticoagulation. No prosthetic valve endocarditis and no implantation of a new permanent pacemaker was reported. No mild, moderate or severe paravalvular regurgitation assessed by echocardiography (evaluated by a core lab) was detected (Figure 2). Implantation of the ALLEGRA THV was associated with an improvement in NYHA class, with 28.6% of patients being in NYHA Class III and IV compared to 79.3% at baseline (Figure 3).
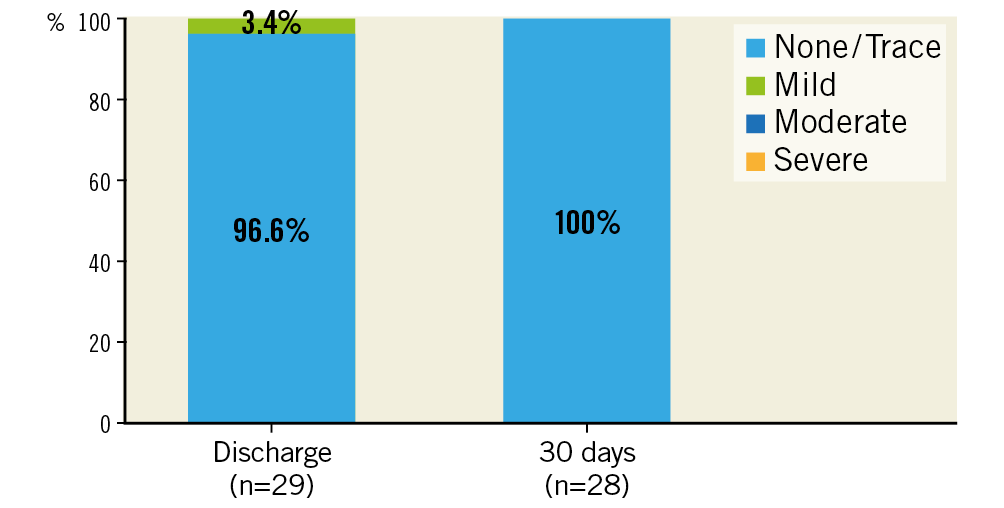
Figure 2. Paravalvular regurgitation at discharge and 30 days.
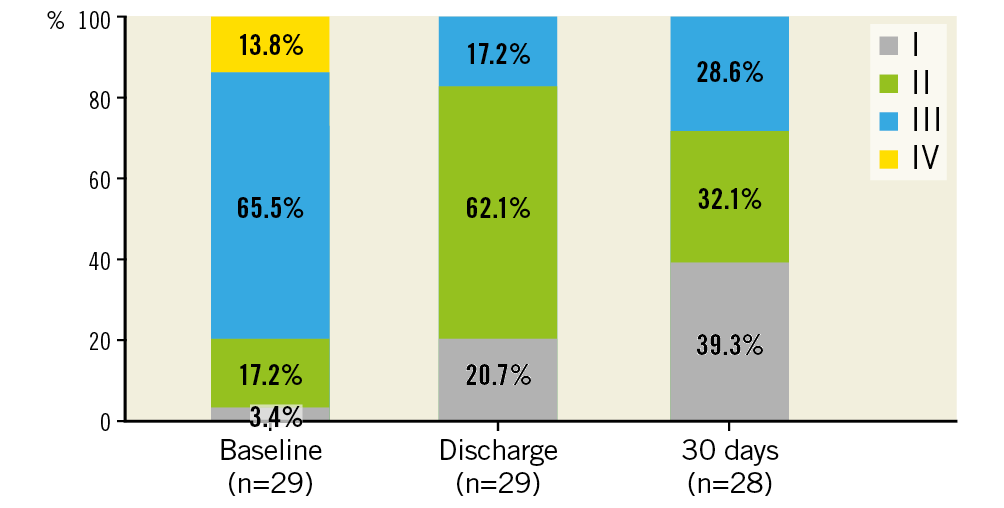
Figure 3. NYHA class at baseline, discharge and 30 days.
Discussion
This study reports the 30-day outcomes of 30 patients with failing aortic bioprostheses undergoing ViV treatment with the self-expanding ALLEGRA THV within the scope of the prospective, multicentre, single-arm VIVALL study.
Even though the STS score suggests an intermediate risk for the VIVALL population (4.5±2.1%), it is worth noting that 90% of the patients were treated with a 23 mm ALLEGRA THV due to the small inner diameters of the surgical bioprostheses, which represents a remarkably high percentage when compared to other published studies11,15,16.
In fact, 80.0% of the surgical valves had an inner diameter of ≤21 mm and 23.3% of ≤19 mm. Only four patients had a surgical bioprosthesis labelled with 25 mm or 27 mm outer diameter (Supplementary Figure 1A). Moreover, 43% of the surgical bioprostheses in the study population were Mitroflow and 6.7% were Mosaic® (Medtronic) prostheses with well-known very small inner diameters.
Small bioprostheses commonly present higher gradients, even in the absence of structural degeneration, and smaller EOAs22. In addition, published data suggest that surgical bioprostheses with externally mounted leaflets and devices with a small inner diameter display an elevated risk for mortality, coronary obstructions and increased gradients across the bioprosthesis after ViV treatment11,23, suggesting that the VIVALL study population represents a challenging group of patients.
Initial malposition occurred in four patients (13.3%), which is comparable to what has been previously reported in the VIVID valve-in-valve registry10. In one patient, an Evolut R was implanted (3.3%), whereas in the other three cases the ALLEGRA THV was retrieved and subsequently implanted in the correct position, resulting in an overall procedural success rate of 96.7%.
The combined secondary endpoint of technical implantation success was achieved in 60% of the patients. At first sight this number looks low but was mostly caused by eight patients with calculated severe PPM (EOAi <0.65 cm2/m2) and an additional two cases in which the EOAi calculation was only based on continuous-wave (CW) Doppler. These were therefore considered as failure in order to be conservative in the analysis. Nevertheless, mean gradients in these eight cases ranged between 5 and 16 mmHg. Low EOAi values were however expected, considering the high number of surgical valves with very small inner diameters. Interestingly, invasive acute outcomes were similar between subgroups depending on their inner diameter derived from MSCT (Supplementary Figure 1B). In addition, 10 patients (33.3%) had stenotic surgical valves and 10 patients (33.3%) had a mixed failure mode, a condition linked to pannus formation usually resulting in higher gradients after ViV treatment. Hence, the mean EOAi in the study population increased from 0.64±0.31 cm2/m2 at baseline to 0.78±0.25 cm2/m2 at discharge after implantation of the ALLEGRA THV.
Notably, the survival rate at 30 days was 100%; no coronary obstruction was detected. Mean gradients at discharge and at 30 days as assessed by an independent echocardiographic core lab (16.1±6.4 mmHg and 14.8±6.5 mmHg, respectively) and evaluated invasively post procedure (11.6±3.7 mmHg) were low for ViV procedures, albeit higher than those seen after TAVI in native aortic valves with the ALLEGRA THV17,18,24 due to the smaller inner diameter of the surgical bioprosthetic valves.
In summary, the data of the VIVALL study demonstrate that treatment of even small surgical prosthetic valves with the self-expanding ALLEGRA THV is feasible and safe and is associated with good haemodynamic and clinical outcomes. To the best of our knowledge, this study reports the lowest rates for mortality (0%), paravalvular regurgitation (all evaluated as none or trace) and implantation of new pacemakers (0%) after 30 days in a ViV environment that have been published so far.
Limitations
This study is a non-randomised single-arm study evaluating TAVI with the ALLEGRA THV System TF in a relatively small number of patients with failing surgical aortic bioprosthetic valves. Although five centres recruited patients, the majority of patients (73.3%) were enrolled at just one site. Subgroup analysis indicates promising haemodynamics after ViV with the ALLEGRA THV; however, robust conclusions cannot be made due to the small sizes of the individual groups.
Conclusions
The 30-day data of the VIVALL study show that the self-expanding ALLEGRA THV can be safely implanted in degenerated surgical bioprostheses, even in those with very small inner diameters, resulting in favourable haemodynamic results and an extremely low rate of paravalvular regurgitation. The particular valve design appears to be an appealing concept for patients for a valve-in-valve treatment in failing surgical aortic bioprostheses.
|
Impact on daily practice Many patients with a failing surgical bioprosthesis have an increased risk for surgical reoperation because of advanced age and additional comorbidities. It is expected that the number of failing surgical bioprostheses will increase significantly in the coming years. TAVI systems with beneficial haemodynamics and proven safety outcomes, such as the ALLEGRA THV System TF, might be of significant benefit to spare elderly patients from redo open heart surgery. |
Funding
This study was funded by New Valve Technology GmbH.
Conflict of interest statement
U. Schäfer is a consultant, proctor and is on the speaker’s bureau for Abbott Vascular, Boston Scientific, Edwards Lifesciences, Medtronic, Gore, JenaValve Technology, and New Valve Technology and has received research support from Abbott Vascular, Boston Scientific, JenaValve Technology, Edwards Lifesciences and New Valve Technology. C. Butter is on the speaker’s bureau for Edwards Lifesciences and Medtronic and has received research support from New Valve Technology. M. Landt has received research support from New Valve Technology. C. Frerker is on the speaker’s bureau and has received travel grants from Abbott Vascular, Boston Scientific, Edwards Lifesciences and Medtronic and has received research support from New Valve Technology. H. Treede is a consultant for and is on the speaker’s bureau for Abbott Vascular, Boston Scientific, Edwards Lifesciences, JenaValve Technology, and Medtronic and has received research support from Abbott Vascular, Boston Scientific, Edwards Lifesciences, JenaValve Technology and New Valve Technology. J. Schirmer is a proctor for Boston Scientific and JenaValve Technology, is on the speaker’s bureau and has received travel grants from Edwards Lifesciences and Medtronic. C. Koban has received research support from New Valve Technology. A. Allali has received research support from New Valve Technology. E. Charitos has received research support from New Valve Technology. T. Schmidt is on the speaker’s bureau and has received travel grants from Edwards Lifesciences, Boston Scientific and Medtronic. L. Conradi is a consultant for Edwards Lifesciences, Boston Scientific, Abbott Vascular, JenaValve Technology; speaker for Edwards Lifesciences, Boston Scientific, Abbott Vascular, Medtronic and JenaValve Technology, has received research support from Abbott Vascular, Boston Scientific, JenaValve Technology, Edwards Lifesciences and New Valve Technology and travel support from Biotronik.




