
Abstract
Aims: We aimed to examine the effectiveness and the optimal technique for transcatheter therapy for residual mitral regurgitation (MR) after MitraClip therapy with the AMPLATZER Vascular Plug II (AVP-II).
Methods and results: Nine patients (mean age, 78±4 years) underwent transcatheter therapy with the AVP-II for residual MR after MitraClip therapy. We examined procedural, in-hospital, and 30-day outcomes. Our technique was successful in all cases, with treatment of different types of residual MR, including paraclip, interclip, and leaflet perforation. MR grade decreased significantly from 4+ to 1+ (p<0.0001), with final residual MR being mild or none in seven patients. Mitral stenosis did not occur with plug placement. The optimal deployment technique for reduction of MR was placement with only one segment on the left atrial side of the mitral valve leaflets (n=8). During clinical follow-up (median 155 days), symptom improvement had occurred in all patients (NYHA class, baseline vs follow-up, 3.2±0.4 vs 2.3±0.8; p=0.01) with mild or no symptoms in six patients. There was no procedural mortality, major adverse event(s), device embolisation, haemolysis or need for cardiac surgery.
Conclusions: For patients with residual MR after MitraClip therapy, this technique may be effective and safe, especially when deployed with only one segment on the left atrial side of the mitral leaflets.
Introduction
Transcatheter mitral valve (MV) repair with the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) is known to be effective and safe in patients at high or prohibitive surgical risk with severe, symptomatic mitral regurgitation (MR)1,2,3. Nonetheless, residual or recurrent MR may occur in 5 to 40% of patients, and has been associated with persistent symptoms and impaired survival3,4. The causes of residual or recurrent MR after MitraClip therapy are variable, with origination next to (“para”) or between clips (“interclip”), or related to anatomic defects (e.g., leaflet perforation). Subsequent transcatheter MitraClip therapy can be difficult due to space constraints and concern for worsening of mitral stenosis (MS), and surgery may be required despite the high-risk nature of these patients4.
Transcatheter occluders are repositionable devices that may overcome the technical challenges associated with treatment of these patients, and therefore should be preferred over repeat MitraClip therapy or high-risk cardiac surgery. Presently, however, data on the use of such devices are limited5,6,7,8,9,10. The AMPLATZER™ Vascular Plug II (AVP-II; St. Jude Medical [now Abbott Vascular], St. Paul, MN, USA) is a relatively low-profile device, which can be delivered with conventional coronary guide catheters. The device is configured with three same-sized diameter segments (i.e., two retention discs and one central waist) that may be effective in these patients. In this study, we aimed to examine the effectiveness and optimal technique for implantation of the AVP-II for the treatment of residual MR in patients with prior MitraClip therapy.
Methods
STUDY POPULATION
Of 261 consecutive patients who underwent MitraClip therapy during the past five years at our hospital (Abbott Northwestern Hospital, Minneapolis, MN, USA), nine patients who underwent transcatheter therapy using the AVP-II for severe, symptomatic residual MR after MitraClip therapy were examined in this study. Each patient was evaluated by a multidisciplinary Heart Team and discussed in the weekly care planning conference. All patients provided informed consent for use of this technique with the AVP-II before the MitraClip procedure, and for use of their medical records for research purposes, in accordance with Minnesota statutes. The study was conducted in accordance with the Declaration of Helsinki and approved by the Allina Institutional Review Board.
PROCEDURE
The procedure was performed under general anaesthesia using a transseptal approach with the support of transoesophageal echocardiography (TEE). MitraClip therapy had been performed as previously described, and significant, residual MR was evident in each patient11.
For patients who had AVP-II placement during the same procedure as MitraClip therapy, the steerable guide catheter was exchanged over a 260 cm Safari™ wire (Boston Scientific, Marlborough, MA, USA) for a 24 Fr DrySeal sheath (W.L. Gore, Flagstaff, AZ, USA), which was placed in the right femoral vein. For patients in whom AVP-II placement was performed in a procedure separate from MitraClip therapy, a standard transseptal puncture from the right femoral vein was performed with placement of an 8 Fr Mullins sheath.
For all patients, an 8.5 Fr, medium-curved Agilis™ catheter (St. Jude Medical [now Abbott Vascular]) with bidirectional steering was placed in the left atrium (LA), followed by insertion of a 5 Fr diagnostic multipurpose (MP) catheter telescoped inside a 6 Fr MP guide. The entire assembly was steered towards the MV, with the targeted area then crossed with a 260 cm, angle-tipped GLIDEWIRE® (Terumo, Somerset, NJ, USA). The 6 Fr MP guide was advanced into the left ventricle (LV). The size of the AVP-II was chosen based on the vena contracta from echocardiography in multiple views on TEE. It is important to note that the boundaries of the colour flow do not indicate the complete edges of the defect. In general, we chose a device diameter that was 30 to 50% larger than the largest diameter measured to minimise the risk of embolisation. The AVP-IIs were deployed in several configurations relative to the mitral leaflets: 1) waist in the coaptation plane of the mitral leaflets and one segment on the LA side and one on the LV side; 2) two segments on the LA side; or 3) two segments on the LV side. Multiple device position configurations were tried in each patient; final positioning was chosen based on improvement in MR. The AVP-II was then decoupled from the delivery cable following confirmation of MR improvement, no worsening of MS, and stability checked with aggressive tug testing. After release, patients were carefully examined for residual MR, and the position of the device was confirmed with TEE and fluoroscopy before extubation. All patients underwent post-procedural TTE for examination of MR and device positioning prior to discharge. All patients were treated with either dual antiplatelet therapy with aspirin (81 to 100 mg daily) and clopidogrel (75 mg daily) for a minimum of three months, or single antiplatelet and anticoagulant therapy. Follow-up was performed in all patients at one month from the procedure, and every six to 12 months thereafter with serial echocardiograms.
STATISTICAL ANALYSIS
Baseline and post-procedural MR severity were defined using standard echocardiographic criteria12. Clinical outcomes during the procedure, hospitalisation, at 30 days, and at last follow-up were examined. Using Mitral Valve Academic Research Consortium (MVARC) criteria, major adverse clinical events were defined as occurrence of death, re-hospitalisation for heart failure, myocardial infarction, stroke, subsequent cardiac surgery, or repeat transcatheter procedure13,14. Data are shown as mean±standard deviation (SD) or median with interquartile range (IQR) where specified. Categorical data were compared using chi-square or Fisher’s exact tests, where appropriate. For normally distributed variables independent t-tests were used to assess group differences. The distributions of continuous, skewed data were compared using a non-parametric Wilcoxon rank-sum test. All statistical analyses were performed with SPSS, Version 25 (IBM Corp., Armonk, NY, USA).
Results
Nine patients with prior MitraClip therapy (mean age, 78±4 years; seven men) underwent transcatheter repair of residual MR with an AVP-II (Table 1). The location of the residual MR was adjacent to the clip in four patients (i.e., paraclip), between clips in four patients (i.e., interclip), and due to leaflet perforation adjacent to a MitraClip that had been placed at an outside institution in one other patient. Elderly age and morbidities were frequent, with a mean Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) of 5.6±5.2% and 7.6±5.9%, for repair and replacement, respectively. Overall, the left ventricular function was preserved in most patients (mean ejection fraction, 52.9±14.8%). In two patients, transcatheter placement of the AVP-II was performed immediately after MitraClip therapy during the same procedure. In seven other patients, the procedure was performed separately, with a median time of 15 months (range, 8 to 22 months) from MitraClip therapy.
PROCEDURAL OUTCOMES
Device implantation was successful in all the patients. All patients received a single AVP-II, with a 12 mm used in six patients, a 16 mm placed in two patients, and a 22 mm in one patient. The size was chosen based on echocardiographic measurement of the vena contracta with adequate oversizing to help ensure device stability. Overall, average residual MR grade decreased significantly from 4+ to 1+ (p<0.0001), with seven patients having mild or no residual MR. In eight patients, the AVP-II was placed with only the one segment in the LA side of the mitral leaflets. In one patient, the device was placed with the waist in the coaptation plane of the mitral leaflets with mild residual MR. No patients had worsening of MS with device implantation (baseline vs post-procedural mean mitral gradient, 3.3±0.7 vs 3.4±1.0 mmHg, p=0.8) (Table 2). For all patients, there were no major adverse events, including death, myocardial infarction, stroke, device-related adverse events, or urgent cardiovascular surgery during hospitalisation. Overall, median length of hospital stay was two days.
PROCEDURAL EXAMPLES
PARACLIP MITRAL REGURGITATION
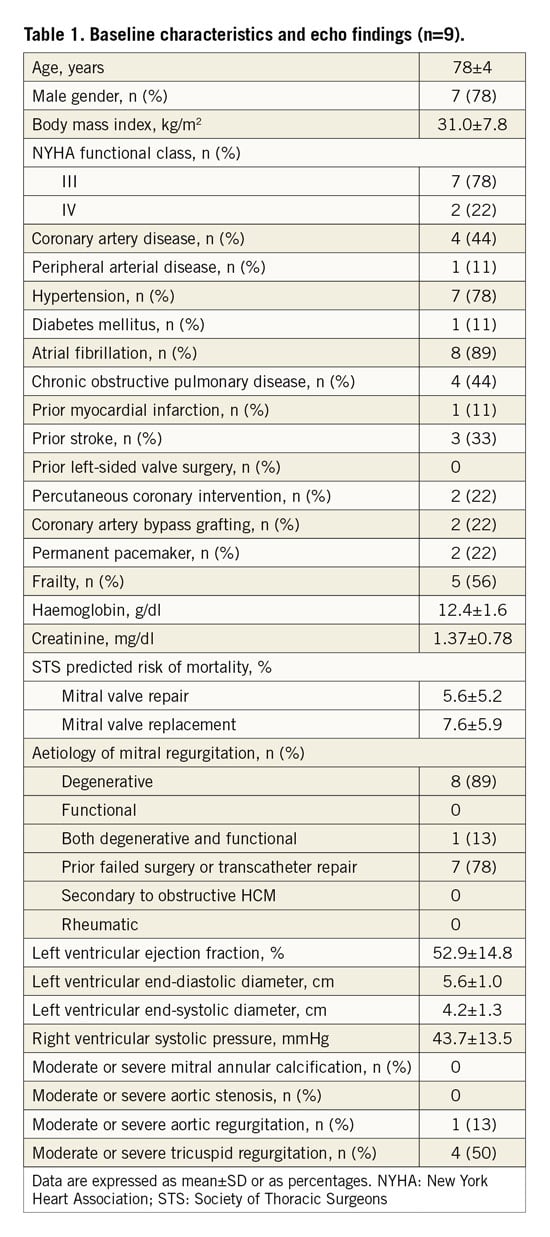
A 79-year-old man underwent MitraClip therapy 25 months previously, which initially achieved a satisfactory result with a single clip on the A2-P2 segments of the MV. However, over the six months prior to re-hospitalisation, he developed recurrent New York Heart Association (NYHA) Class III to IV symptoms, and TEE showed eccentric, severe MR due to a new flail A1-P1 segment (Figure 1A). Following a Heart Team evaluation, MitraClip therapy was performed with placement of the additional clip as far lateral in the commissure as possible. However, the flail segment was further lateral than the deployed additional clip, with severe residual MR (i.e., paraclip) (Figure 1B). We selected a 16 mm AVP-II according to TEE imaging. First, the waist of the device was placed in the coaptation plane of the MV leaflets (Figure 1C); severe residual MR was still present. Subsequently, the plug was placed with only the proximal segment in the LA side of the mitral leaflets, and both the waist and the distal segment (i.e., two segments) in the LV side (Figure 1D). The MR grade was effectively reduced to trivial, and the mean mitral gradient was 2 mmHg (Figure 1E, Figure 1F). The patient had significant clinical improvement. The MR reduction remained stable with NYHA Class I to II symptoms at the end of follow-up.
Figure 1. Transcatheter therapy with the AVP-II for paraclip mitral regurgitation. A) Transoesophageal echocardiography (TEE) demonstrating the presence of severe recurrent mitral regurgitation (MR; arrow). B) TEE demonstrating the presence of severe residual MR (arrow) after additional clip deployment. C) TEE showing the first position of the deployed 16 mm AVP-II (arrow). D) Post-procedural TEE showing the final position of the deployed AVP-II (arrow). E) Post-procedural TEE showing trivial residual MR (arrow) after AVP-II deployment. F) Doppler echocardiography showing the mitral gradient. LA: left atrium; LV: left ventricle
INTERCLIP MITRAL REGURGITATION
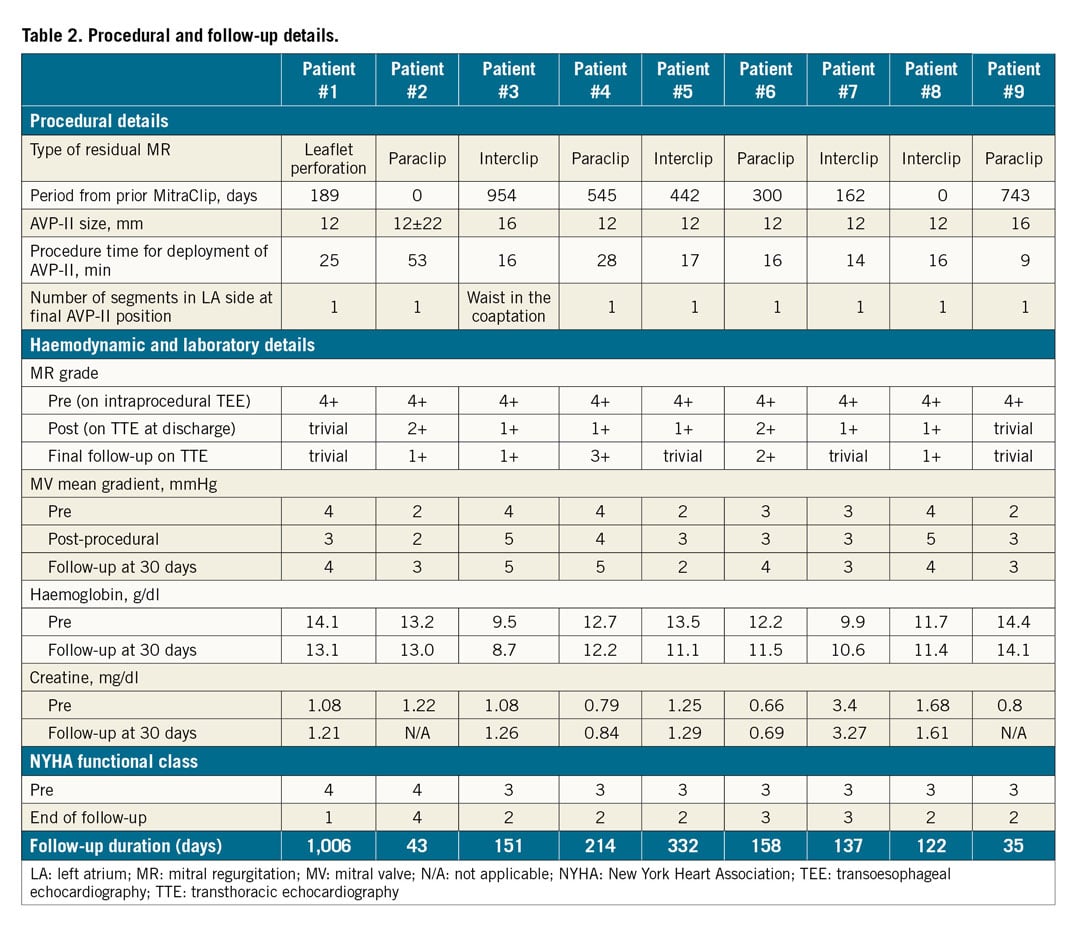
A 73-year-old man underwent MitraClip therapy 30 months before, with successful placement of two clips on the A2-P2 segments of the MV. He became hospitalised for severe heart failure, and TEE showed severe recurrent MR from a new flail segment (Figure 2A, Figure 2B). Following a Heart Team evaluation, the third MitraClip was deployed at the new flail segment. Following deployment of the third clip (Figure 2C), TEE demonstrated residual MR between the first and second clips (i.e., interclip) (Figure 2D), and not enough room for an additional clip. An angle-tipped 0.035” Glidewire and a 6 Fr MP guide catheter were carefully passed between the first and second previously placed clips (Figure 2E). A 16 mm AVP-II was then deployed in this area as the measurement of the interclip defect using 3D-TEE showed that the distance between the two clips was 9 mm (Figure 2B, Figure 2F, Figure 2G). Following deployment of the plug, the residual MR was only mild (Figure 2H), and the mean mitral gradient was 3 mmHg. The MR reduction remained stable with NYHA Class II symptoms at the end of follow-up.
Figure 2. Transcatheter therapy with the AVP-II for interclip mitral regurgitation. A) Transoesophageal echocardiography (TEE) demonstrating the presence of severe mitral regurgitation (MR; arrow). B) Three-dimensional TEE showing the new flail segment (arrow) and two previously placed MitraClips (arrowheads). The measurement of the interclip defect showed that the distance between the two clips was 9 mm. C) Fluoroscopy demonstrating deployment of the third MitraClip (arrow) and two previously placed MitraClips (arrowheads). D) TEE demonstrating the presence of severe residual MR (arrow) after additional clip (arrowhead) deployment. E) Fluoroscopy demonstrating crossing of the Glidewire and the 6 Fr multipurpose guide catheter between two previously placed MitraClips (arrow), F) Fluoroscopy demonstrating deployment of the 16 mm AVP-II plug (arrow). G) Post-procedural three-dimensional TEE showing the placement of the third MitraClip (arrowhead) and the AVP-II (arrow). H) Post-procedural TEE showing mild residual MR (arrow) after AVP-II deployment (arrowhead). LA: left atrium; LV: left ventricle
LEAFLET PERFORATION
An 82-year-old man had persistent, severe symptoms of dyspnoea at six months after a MitraClip procedure performed elsewhere. TEE showed severe MR due to perforation in the anterior leaflet (Figure 3A, Figure 3B). He was initially considered for surgery but was declined by our Heart Team due to multiple prior coronary artery bypass grafting and severe comorbidities. We first deployed an additional MitraClip at the A2-P2 position lateral to the prior clip, which had been placed in the A3-P3 segments. This new clip placement was performed to create a stable landing zone for implantation of an AVP-II (Figure 3C). Following placement of this second clip, severe MR in the perforation remained (Figure 3D). Using the aforementioned techniques, we then deployed a 12 mm AVP-II, with two segments of the occluder in the LV side of the MV. This positioning was chosen to minimise extrusion of the occluder into the left ventricular outflow tract (LVOT). Following deployment, there was trivial MR and no LVOT obstruction (Figure 3E-Figure 3G). The mean mitral gradient was 3 mmHg. Two years after the procedure, he remained asymptomatic. Echocardiography showed minimal residual MR and no device dislocation (Figure 3H).
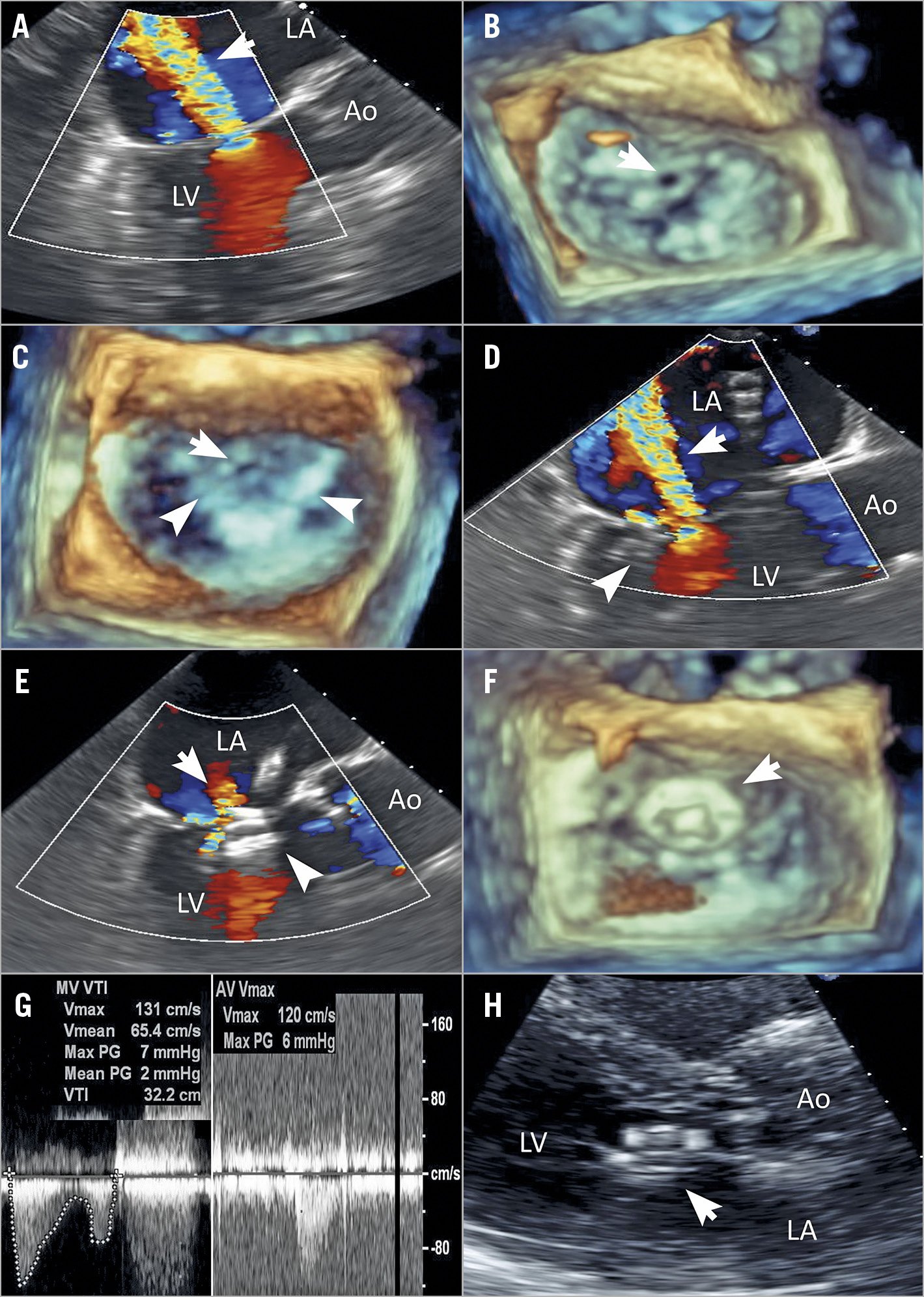
Figure 3. Transcatheter therapy with the AVP-II for leaflet perforation. A) Transoesophageal echocardiography (TEE) demonstrating the presence of severe recurrent mitral regurgitation (MR; arrow). B) Three-dimensional TEE showing a large perforation in the anterior leaflet (arrow). C) Three-dimensional TEE showing the presence of a landing zone (arrowheads) for implantation of an AVP-II and a leaflet perforation (arrow). D) TEE demonstrating the presence of severe residual MR (arrow) after additional clip deployment. E) Post-procedural TEE showing trivial residual MR (arrow) after 12 mm AVP-II deployment (arrowhead). F) Post-procedural three-dimensional TEE showing the placement of the AVP-II (arrow). G) Doppler echocardiography showing the mitral gradient, the peak left ventricular outflow tract (LVOT) velocity and the LVOT gradient. H) Transthoracic echocardiography demonstrating no AVP-II dislocation (arrow). Ao: aorta; LA: left atrium; LV: left ventricle
FOLLOW-UP EVALUATION
For all patients, the median follow-up duration was 155 (IQR 133, 244) days. There were no incidences of device embolisation, and there was no evidence of haemolysis throughout the entire study period (Table 2). One patient died from pneumonia. There was persistent reduction in MR, and no worsening of MS (mean gradient, 3.3±0.7 vs 4.9±2.2; p=0.065). Symptom improvement had occurred in all patients (NYHA class, baseline vs follow-up, 3.2±0.4 vs 2.3±0.8; p=0.013) with mild or no symptoms in six patients.
Discussion
The purpose of the present study was to examine the effectiveness of AVP-II deployment for residual MR after MitraClip therapy. Procedure success was achieved with deployment of a single AVP-II in all cases. In seven patients, residual MR grade decreased significantly to mild or trivial. In the other two patients, residual MR grade remained only mild to moderate. Symptoms improved in all patients with NYHA Class I or II symptoms in six patients. No patients had worsening of MS and incidence of device embolisation or haemolysis with device implantation. Moreover, there were no associated deaths or adverse events. Taken together, these findings suggest that recurrent MR after MitraClip therapy may be successfully treated with transcatheter therapy using an AVP-II and may lead to satisfactory patient outcomes.
While MitraClip therapy is an established treatment for severe symptomatic MR, residual or recurrent MR can occur and impair long-term prognosis4. Although the effectiveness of additional clips has also been shown, there are cases in which reintervention with the MitraClip is difficult for anatomical reasons (i.e., not enough room for an additional clip, or leaflet perforation). In the present study, we found implantation of an AVP-II to be effective in a patient with different types of residual MR that can occur after MitraClip therapy.
The short-term outcomes of a similar technique using the AMPLATZER™ Duct Occluder II (ADO-II; St. Jude Medical) and the GORE® CARDIOFORM Septal Occluder (W.L. Gore) have been described as being favourable, though do include device dislocation and haemolysis6,8. The AMPLATZER Atrial or Ventricular Septal Duct (ASD or VSD) Occluders have also been used5. Selection of the occluder device depends on the location, size, and mechanism of the residual or recurrent MR.
We prefer the AVP-II, which offers considerable stability due to its three-segment construction, which also enhances sealing of the regurgitation. The AVP-II uniquely permits adjustment of the position of the device segments. When deployed with two segments on the LV side, relief of MR and symptoms occurred in all such treated patients. The retention discs help to prevent device dislocation or embolisation, neither of which was observed in our patients, with some patients having >2 years of follow-up after deployment. The risk of haemolysis, in comparison to other devices, is relatively lower due to the small calibre of the nitinol mesh8,15,16,17. Finally, the AVP-II is relatively less expensive and has a broad range of sizes. Certainly, while the AVP-II may be highly effective, evidence of long-term durability on all devices remains unavailable, and further follow-up examinations are required. It is also important to note that optimal antithrombotic therapeutic regimens of antiplatelet and anticoagulant therapy in these patients have not been established.
Of the 261 patients who underwent MitraClip therapy during the study period, residual or recurrent severe MR was observed in 13%. Of those with severe residual or recurrent MR, repeat MitraClip implantation was used in 34%, surgery in 25%, and medical management in 41%. This experience mostly predates the availability of current MitraClip devices such as the XTR, which may or may not have lower rates of residual MR. Overall, our technique with the AVP-II was used in only 2.6% of the total number of patients who underwent a MitraClip procedure during the same period. This technique with the AVP-II is not common but can be performed in limited cases with careful patient selection, and only after repeat MitraClip therapy or surgery is it considered through a multidisciplinary evaluation. The use of this technique should be considered only a bail-out strategy and should only be performed by experienced operators.
Limitations
This study was a single-centre and retrospective observation study in a small case series of highly selected patients. Our findings are those of a single-centre tertiary care facility, may be institution-specific, and therefore challenges in generalisability may exist. Further validation with larger series, and more long-term data to support the use of the device in these patients are needed.
Conclusions
Transcatheter therapy with placement of an AVP-II for residual or recurrent MR after MitraClip therapy may be effective and safe, especially when deployed with only the one segment on the LA side of the mitral leaflets.
|
Impact on daily practice Residual mitral regurgitation (MR) after MitraClip therapy can be associated with persistent symptoms and impaired survival. Subsequent MitraClip therapy can be difficult due to space constraints and concern for worsening of mitral stenosis. Also, some patients may require surgery despite the high surgical risk. Our findings suggest that recurrent MR after MitraClip therapy may be successfully treated with transcatheter therapy using the AVP-II and may lead to satisfactory patient outcomes. |
Conflict of interest statement
R. Bae has received a speaker’s fee from Abbott Vascular. P. Sorajja has received research grants, consulting fees and speaker fees from Edwards Lifesciences, Boston Scientific, Medtronic, and Abbott Vascular. The other authors have no conflicts of interest to declare.




