
Abstract
Aims: Interventional mitral repair techniques have evolved as safe and effective treatment options for patients with functional mitral regurgitation (FMR) at high or prohibitive surgical risk. Of the techniques available, the MitraClip device and Cardioband mitral repair system have been used most commonly. However, a direct comparison of the two devices, examining their effectiveness at reducing MR, reducing symptoms, and extending life expectancy, has not yet been performed. For this purpose, we compared the outcome of patients after direct annuloplasty by the Cardioband system with patients after edge-to-edge therapy with the MitraClip device in a propensity score-matched analysis.
Methods and results: We collected data concerning 123 consecutive patients who were treated with the Cardioband device and 455 consecutive patients treated with the MitraClip from five experienced European centres. Propensity score matching was performed, resulting in two groups with 93 patients each – with no significant differences regarding baseline demographic parameters – who underwent standardised 2D transthoracic echocardiography with assessment at baseline and clinical follow-up at 12 months. The success rate, defined as a reduction of MR to grade 2 or lower, was high in both groups (MR ≤2: MitraClip: 86%, Cardioband: 77%, p=0.18). The Cardioband was better at reducing heart failure symptoms (NYHA ≤II: 88%) than the MitraClip (75%) procedure (p=0.046) at 12-month follow-up. All-cause rehospitalisation and mortality within 12 months were lower in Cardioband patients (mortality: OR 0.30, CI: 0.09-0.98, p=0.032; rehospitalisation: OR 0.57, CI: 0.28-0.97, p=0.03).
Conclusions: The MitraClip and the Cardioband procedures effectively reduce MR and heart failure symptoms. However, patients undergoing the Cardioband procedure showed a more pronounced improvement with regard to functional NYHA class, rehospitalisation, and mortality, compared to patients undergoing the MitraClip procedure.
Introduction
Currently, transcatheter interventions are increasingly being used for mitral valve repair, offering a new treatment option to patients with mitral regurgitation (MR) who have been deemed inoperable or at high surgical risk1,2,3. The most widely accepted and performed transcatheter therapy is edge-to-edge treatment using the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA), which accounts for more than 80,000 commercial implants since 20084. Together with the Carillon® Mitral Contour System® (Cardiac Dimensions Inc., Kirkland, WA, USA), the Cardioband (Edwards Lifesciences, Irvine, CA, USA), which received a CE mark in 2015, is the second most widely used transcatheter mitral valve repair (TMVR) system, with 500-1,000 implants each5,6.
For the MitraClip (MC) procedure, three randomised studies exist, whereas there are no randomised controlled trials (RCTs) for annuloplasty with the Cardioband (CB) system. In 2015, the EVEREST II trial showed similar mortality rates in patients with predominantly degenerative MR five years after surgical or edge-to-edge therapy7, but higher re-operation rates during the first year in the edge-to-edge group. The MITRA-FR RCT showed a similar outcome between conservative and interventional treatment of functional MR (FMR), whereas the COAPT trial revealed both a symptomatic and prognostic benefit for catheter-based treatment of patients, compared to medical therapy8,9. Differences in outcome between the MITRA-FR and COAPT trials have been discussed, and the consensus is that left ventricular end-diastolic volume (LVEDV), left ventricular ejection fraction (LVEF), as well as effective regurgitant orifice area (EROA), might be important predictors of outcome10.
Direct percutaneous annuloplasty with the CB system is a relatively new technique for TMVR. Annuloplasty is the most commonly performed surgical repair treatment for MR, and is often used as a stand-alone procedure to improve coaptation in FMR11. The CB system is a direct percutaneous annuloplasty device that is implanted in the beating heart on the posterior annulus by using fluoroscopic and transoesophageal echocardiographic (TEE) guidance. Following implantation, the device contracts to reduce the annulus dimensions and improve leaflet coaptation and MR. The feasibility and CE-mark study showed excellent results after CB therapy in FMR patients5, but the results of larger endpoint trials are not yet available. Whether transcatheter edge-to-edge therapy or transcatheter annuloplasty is the better treatment option has also not yet been evaluated. For this purpose, we compared the outcome of patients after direct annuloplasty by the CB system with patients after edge-to-edge therapy with the MC device in a propensity score-matched analysis.
Methods
We analysed data from 123 consecutive CB patients from five European centres (Milan, Zurich, Cologne, Hamburg, and Bonn) and matched them with 455 consecutive MC patients with a propensity score-matching method (Figure 1). The procedures were performed between 2013 and 2018; each centre contributed at least 19 CB patients. After propensity score matching, 93 patients with FMR remained in each group. Device and implant techniques for both devices have been described in detail elsewhere5,12. Five different centres enrolled adult individuals at high surgical risk with symptomatic MR despite being given optimal medical therapy, e.g., cardiac resynchronisation therapy (CRT). All patients underwent preprocedural TEE. CB patients additionally had cardiac computed tomography (CT) to assess the anatomical feasibility of the procedure. CT scanning was used to determine the size of the mitral annulus and to plan the procedure. The primary efficacy endpoints included reduction of MR and the New York Heart Association (NYHA) class at 12 months after the TMVR. Secondary endpoints comprised length of hospital stay following the index procedure, mortality, and rehospitalisation 12 months after TMVR according to previously published endpoint definitions and recommendations for MR assessment13,14.
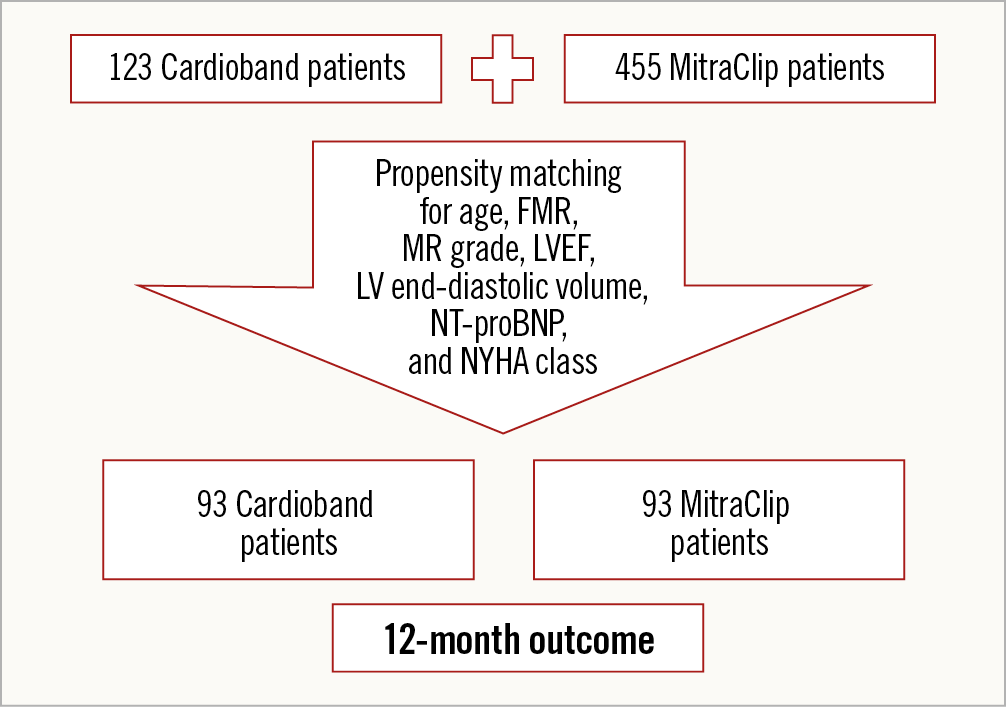
Figure 1. Study flow chart.
STATISTICAL ANALYSIS
Statistical analysis was conducted using SPSS, Version 24.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as the mean±SD or as the median (IQR, Q1-Q3) and categorical variables are expressed as percentages. Univariable comparisons (differences between pre- and post-values) were performed with the Student’s paired t-test for continuous normally distributed data, which was tested by the Shapiro-Wilk normality test. A p-value of 0.05 or less was considered statistically significant; reported p-values are two-sided. To minimise differences amongst the groups due to confounding variables, propensity score matching was performed. For all FMR patients, the propensity score was obtained by a binary logistic regression model with the type of mitral valve intervention (MC versus CB) as a binary outcome, and variables deemed to be associated with clinical outcome after TMVR (age, MR grade, LVEF, LVEDV, NT-proBNP, and NYHA class) were included as predictors. Patients were matched 1:1 with a maximum matching tolerance of 25% to eliminate bias in the observed confounder. Standardised differences were reported for baseline characteristics. A univariate analysis was performed with one-year mortality and one-year rehospitalisation as a dependent variable. The logistic regression model was built by selecting baseline variables of clinical interest and/or satisfaction with the entry criterion of p<0.1 in the univariate analysis.
Results
PATIENT COHORT AND ACUTE RESULTS AFTER TMVR
Patients were on average 75.9±6.3 years old and at moderate to high surgical risk, with a mean logistic EuroSCORE of 18.5±14.6%. Propensity score matching revealed two groups of 93 patients each who showed no significant differences regarding baseline demographic parameters, including age (CB: 75.3±5.8 years vs MC: 76.6±6.6 years, p=0.11), logistic EuroSCORE (CB: 19.9±12.7% vs MC: 15.6±17.9%, p=0.2), LVEF (CB: 38.1±12.2% vs MC: 38.7±13.0%, p=0.81), baseline NT-proBNP level (CB: 4,261±4,085 pg/ml vs MC: 5,343±5,465 pg/ml, p=0.17), and systolic pulmonary artery pressure (sPAP) (CB: 43.3±15.4 mmHg vs MC: 40.8±12.8 mmHg, p=0.23) (Table 1).
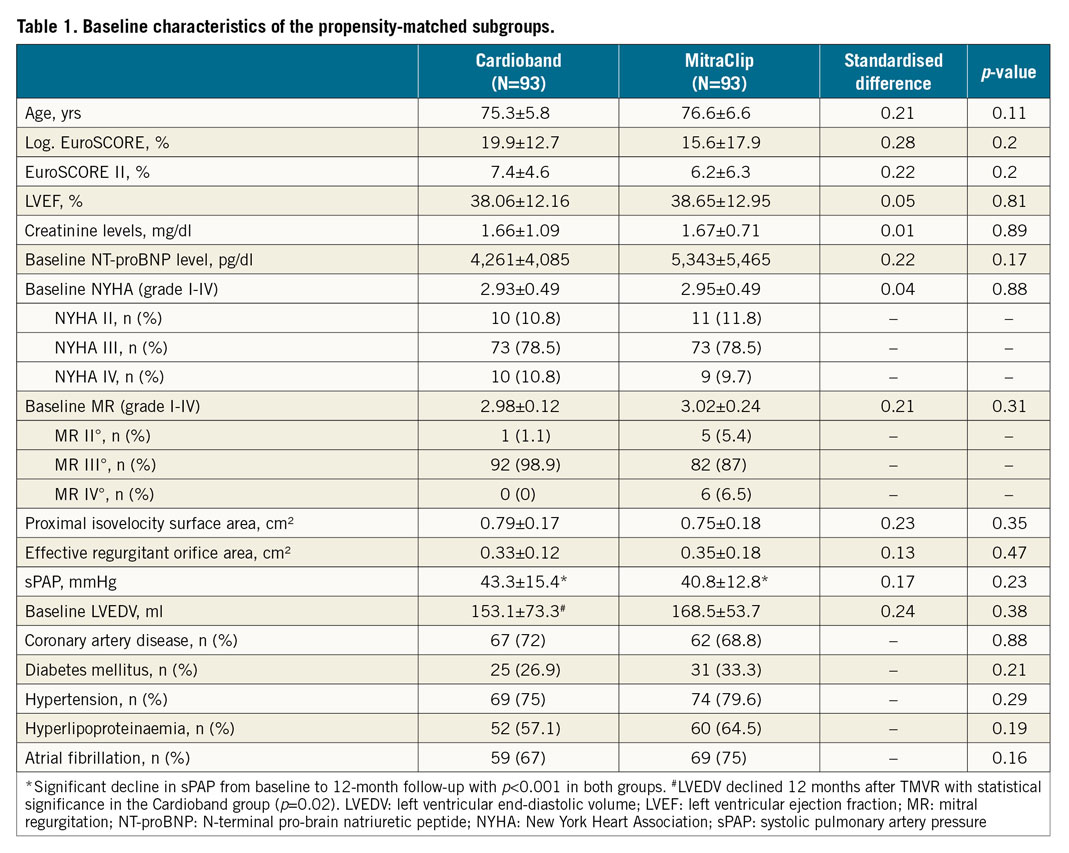
All patients underwent standardised two-dimensional transthoracic echocardiography according to actual recommendations14. Baseline MR grade was comparable between the two groups (CB 2.98±0.12, MC 3.02±0.24, p=0.31). This was also true of the baseline EROA in both groups (CB: 0.33±0.12 cm² vs MC: 0.35±0.18 cm², p=0.47). Additionally, the baseline NYHA class was not significantly different between the groups (CB: 2.93±0.49 vs MC: 2.95±0.49, p=0.88).
No periprocedural deaths occurred. Length of hospital stay was significantly shorter after the CB procedure compared to the MC procedure (CB: 7.9±4.6 days vs MC: 11.7±7.6 days, p<0.001). Three patients in the MC group (3.2%) and one patient in the CB group (1.1%) died within the first 30 days after the procedure. There were no major device- or procedure-related serious adverse events in either group.
ONE-YEAR RESULTS AFTER TMVR
The success rate was defined as a reduction of MR to grade 2 or lower and was high in both groups (MR ≤2: MC: 86%, CB: 77%, p=0.18). Heart failure symptoms were more frequently reduced to NYHA class ≤II in the CB (88%) than in the MC (75%) group (p<0.05) at 12-month follow-up. Absolute MR grade was 1.94±0.73 12 months after CB procedure and 1.82±0.67 12 months after MC treatment (p=0.24). Mean mitral valve gradient was significantly higher in MC patients (3.26±1.51 mmHg) compared to CB patients (1.71±0.99 mmHg, p<0.001). One year after TMVR, LVEF and NT-proBNP levels were not significantly different between the two procedures (LVEF CB: 39.5±12.3% vs MC: 43±14%, p=0.36; NT-proBNP CB: 4,175±4,388 pg/ml vs MC: 4,139±5,141 pg/ml, p=0.98).
The LVEDV 12 months after TMVR declined in both groups, but this reduction was statistically significant only in the CB group (MC baseline LVEDV: 168.5±53.7 ml, 12 months: 160.4±68.7 ml, p=0.42; CB baseline LVEDV: 153.1±73.3 ml, 12 months: 140.4±70.9 ml, p=0.02). There was no significant difference between the groups at baseline as well as at 12 months with regard to LVEDV (Table 1, Table 2). One year after the procedure, sPAP was assessed by Doppler-derived echocardiography. No significant difference was seen between the groups (CB: 33.9±11.7 mmHg vs MC: 37.7±14.5 mmHg; p=0.17) (Table 2), but there was a significant decrease in sPAP from baseline to 12 months in both groups (p<0.001) (Table 1, Table 2).
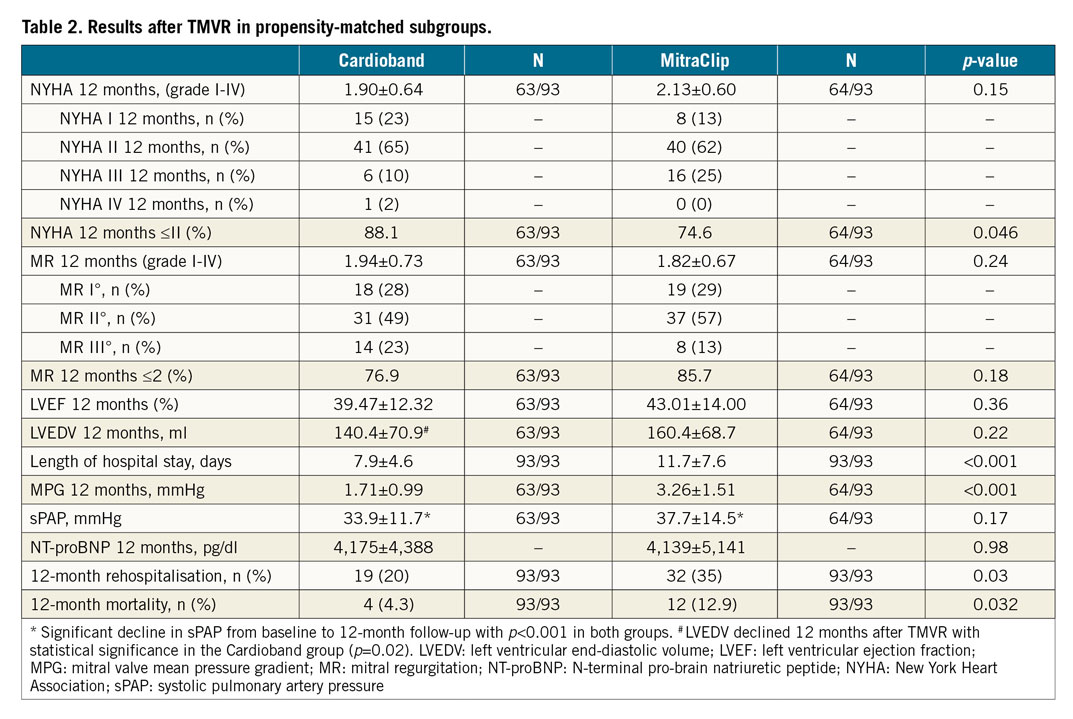
All-cause rehospitalisation and mortality within the first 12 months after the procedure were lower in CB patients (mortality: 4.3% vs 12.9%, OR 0.30, CI: 0.09-0.98, p=0.03 [Figure 2]; rehospitalisation: 20% vs 35%, OR 0.57, CI: 0.28-0.97, p=0.03 [Table 2]).
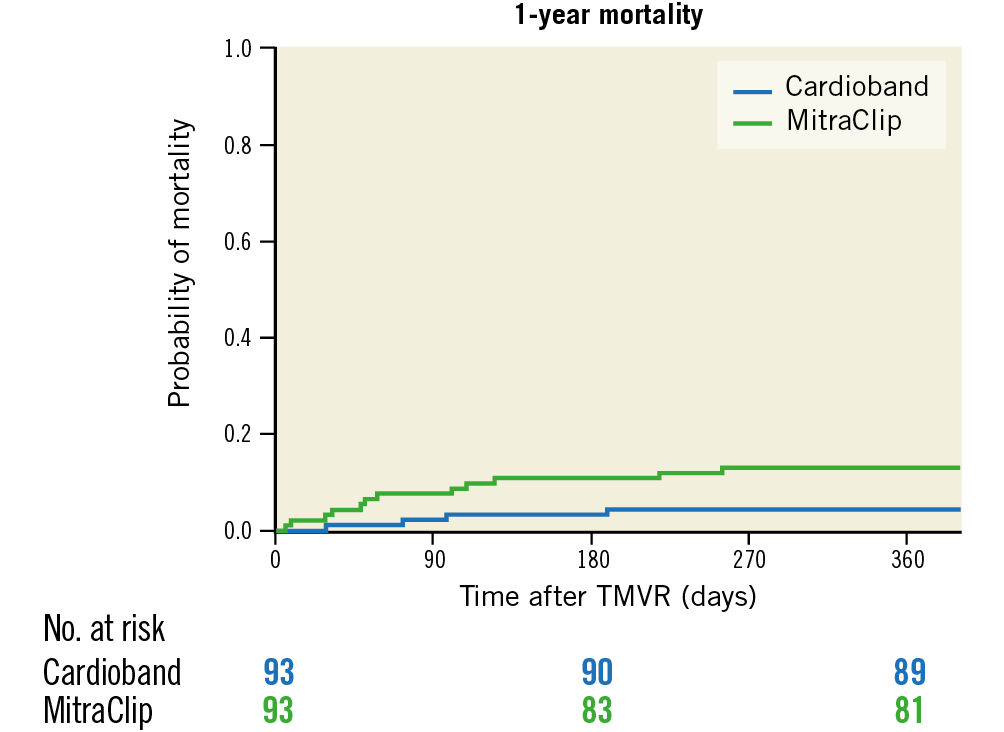
Figure 2. Kaplan-Meier estimation of survival one year after Cardioband (blue) and MitraClip (green) implantation, p=0.03.
The difference in rehospitalisation rate was especially pronounced in patients with an LVEF below 30% (17.9% vs 52.2%; OR 0.26, CI: 0.07-0.96, p=0.02) (Figure 3), whereas only a trend towards lower mortality was seen in patients with severely depressed LVEF treated with the CB system (0% vs 17.4%; p=0.061).
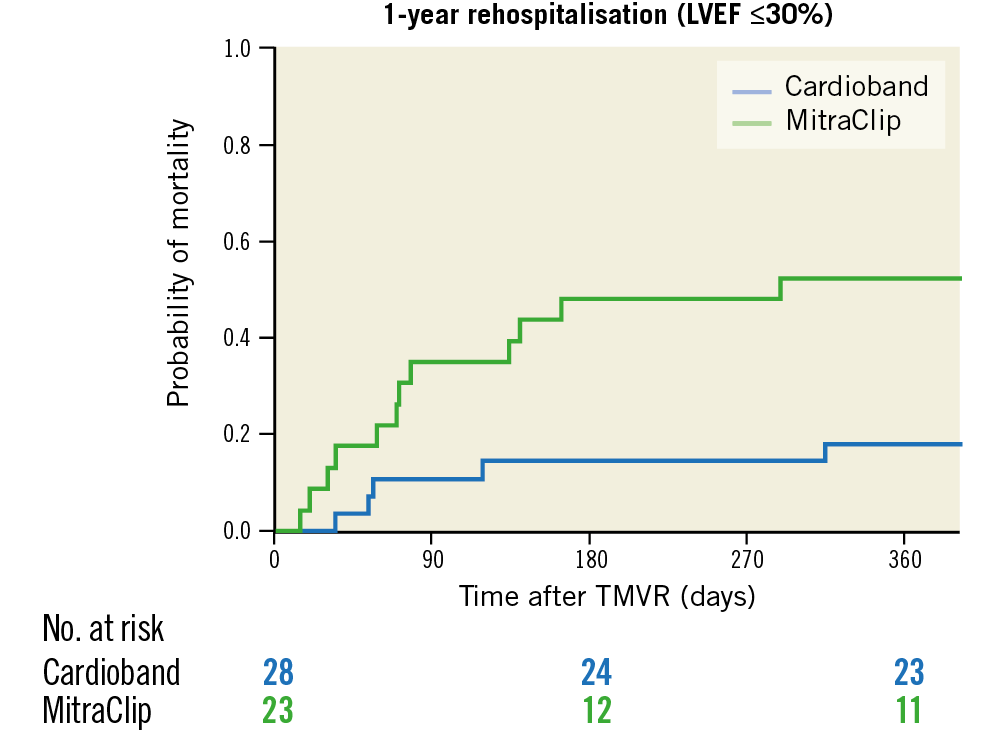
Figure 3. Kaplan-Meier estimates of one-year rehospitalisation after Cardioband (blue) and MitraClip (green) implantation for patients with left ventricular ejection fraction (LVEF) ≤30%, p=0.01.
Logistic EuroSCORE, LVEF, age, EROA, baseline creatinine level, baseline tricuspid regurgitation (TR), baseline sPAP, NT-proBNP, age, baseline LVEDV, and baseline and 12-month mitral valve mean pressure gradient showed no effect on one-year mortality in a univariate variance analysis. Device (p=0.037) and baseline creatinine level (p=0.076) were employed in a logistic regression analysis, which revealed that only the type of device was an independent predictor for mortality (OR 0.3, 95% CI: 0.09-0.97, p=0.044). The same results were obtained for the one-year rehospitalisation rate, where the type of device used was the only independent predictor (OR 0.49, 95% CI: 0.25-0.94, p=0.036).
Discussion
Treatment of FMR with TMVR shows promising periprocedural safety and favourable midterm results with regard to MR reduction, heart failure symptoms, rehospitalisation, and mortality. The length of hospital stay, rehospitalisations, and mortality rates were more favourable after the CB procedure, compared to patients who received the MC. The difference in the one-year rehospitalisation rate was especially pronounced in patients with severely depressed LVEF below 30% (CB: 17.9% vs MC: 52.2%; p=0.016).
PATIENT COHORT AND ACUTE PROCEDURAL SAFETY
The patients in this cohort were at moderate to high perioperative risk and were deemed unsuitable or inappropriate for mitral valve surgery. The average age and risk scores of our patients were comparable to previous trials for transcatheter treatment of functional MR, such as COAPT or MITRA-FR8,9. Regarding LVEDV and EROA, patients of our propensity-matched cohort were comparable more to those in MITRA-FR than to those in COAPT (Table 1). Four patients (2.2%) died within the first 30 days after the procedure, which is comparable to the recently published results of the COAPT trial (2.3% within 30 days)9. Maisano et al reported a 30-day mortality rate after CB implantation of 6.5% in 31 implanted patients5, and MITRA-FR found a rate of 2.7% for 30-day mortality8. Mean hospital stay was not reported in the COAPT or MITRA-FR trials, but the TRAMI registry reported a median hospital stay of 9 (6.0-15.0) days15. In our study, patients were discharged after an average of 7.9 days if treated with the CB and 11.7 days when treated with the MC. It remains unclear why MC patients needed to stay almost four days longer in the hospital.
ONE-YEAR RESULTS AFTER TMVR
The degree of residual or recurrent MR and subsequent heart failure symptoms are key parameters of follow-up for TMVR patients. In the COAPT trial, 94.8% of patients had an MR grade ≤2 after 12 months and 72.2% were in NYHA class ≤II, whereas the MITRA-FR trial found that 91.2% of patients at discharge had an MR grade ≤2 and 71% had an NYHA class ≤II one year after TMVR8,9. ACCESS-EU and TRAMI reported similar findings15,16. In the CE-mark study for the CB device, 79% were in NYHA class ≤II and 95% showed only mild-to-moderate MR (grade 1-2) at 12-month follow-up17.
The functional NYHA assessment after 12 months in our cohort is in line with other studies. However, within our cohort, MC patients had more heart failure symptoms than CB patients, although three quarters of the MC patients were still in NYHA class I or II after one year. This result is slightly better than the results in the COAPT and MITRA-FR trials. Vice versa, MC patients showed a trend towards better reduction of MR than CB patients after 12 months, but the difference was not statistically significant (MR ≤2: MC: 86%, CB: 77%, p=0.18). The reduction in MR in our cohort ranges in the bottom third of previously published data, but MR assessment following the CB or MC procedure is complex and subject to several pitfalls. The CB patients generally display lower mean mitral valve gradient and better LV remodelling. We speculate that this could be due to the direct annuloplasty effect of the device, which may in turn more directly influence LV myocardial performance and wall stress. This effect might also be responsible for the slightly better improvement in heart failure symptoms and rehospitalisation following CB implantation and, in turn, may also drive the better prognosis that we have seen in our matched cohorts. Future imaging and outcome studies with higher patient numbers and a randomised design are warranted.
Limitations
First, the multicentric character of our registry can lead to inaccuracies. There was no core lab adjustment. Second, propensity score matching is not comparable to a randomised study design. Unknown bias such as tenting height, tethering or selection preferences of the operators was not taken into account. However, this study sets the stage for a large-scale analysis, comparing different TMVR strategies for patients with functional MR.
Conclusions
The MitraClip and the Cardioband procedures are effective alternatives for the treatment of patients with symptomatic FMR and a high surgical risk. Both procedures reduce symptoms and improve functional capacities. However, the patients undergoing the Cardioband procedure showed a pronounced improvement regarding the functional NYHA class, rehospitalisation rate, and mortality rate when compared to patients undergoing the MitraClip procedure. Patients with severely depressed LVEF especially benefited from annuloplasty with the Cardioband system compared to the MitraClip edge-to-edge treatment. Long-term and randomised studies are required to confirm these preliminary findings.
|
Impact on daily practice Our data indicate that MitraClip and Cardioband procedures are safe and effective in patients with functional mitral regurgitation. However, patients undergoing the Cardioband procedure may profit from pronounced improvement with regard to rehospitalisation and mortality compared to patients undergoing the MitraClip procedure. |
Acknowledgements
We thank Dr Meghan Campbell for careful reading of the manuscript. We also thank Ms. Theresa Wißt for her systematic data acquisition.
Conflict of interest statement
M. Weber has received travel grants from Edwards Lifesciences and honoraria for advisory boards from Abbott. M. Taramasso is a consultant for Abbott Vascular and has received speaker honoraria from Edwards Lifesciences. R. Pfister has received a travel grant from Abbott. H. Alessandrini has received travel grants from Abbott Vascular and Edwards Lifesciences and honoraria for advisory boards from Edwards Lifesciences. A. Latib has served on advisory boards for Abbott and has been a consultant to Edwards Lifesciences and Abbott and receives research grants from Abbott Vascular and Edwards Lifesciences. P. Denti is a consultant for Abbott Vascular, has received speaker honoraria from Edwards Lifesciences and has been a consultant for Valtech. K.H. Kuck has been a consultant for Abbott and Edwards Lifesciences, has an economic relationship with Valtech and has received research grants from Abbott Vascular and Edwards Lifesciences. F. Maisano has been a consultant for and received institutional grants from Valtech and Edwards Lifesciences, is a shareholder of Valtech Cardio and receives royalties from Edwards Lifesciences. S. Baldus has received speaker’s honoraria as well as travel and grant support from Edwards Lifesciences. G. Nickenig has received research funding, participated in clinical trials and received honoraria for lectures or advisory boards from Abbott and Edwards Lifesciences. The other authors have no conflicts of interest to declare.




