-
PDF
- Split View
-
Views
-
Cite
Cite
Gorav Batra, Leif Friberg, David Erlinge, Stefan James, Tomas Jernberg, Bodil Svennblad, Lars Wallentin, Jonas Oldgren, Antithrombotic therapy after myocardial infarction in patients with atrial fibrillation undergoing percutaneous coronary intervention, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 4, Issue 1, January 2018, Pages 36–45, https://doi.org/10.1093/ehjcvp/pvx033
Close - Share Icon Share
Abstract
Optimal antithrombotic therapy after percutaneous coronary intervention (PCI) in patients with myocardial infarction (MI) and atrial fibrillation is uncertain. In this study, we compared antithrombotic regimes with regard to a composite cardiovascular outcome of all-cause mortality, MI or ischaemic stroke, and major bleeds.
Patients between October 2005 and December 2012 were identified in Swedish registries, n = 7116. Landmark 0–90 and 91–365 days of outcome were evaluated with Cox-regressions, with dual antiplatelet therapy as reference. At discharge, 16.2% received triple therapy (aspirin, clopidogrel, and warfarin), 1.9% aspirin plus warfarin, 7.3% clopidogrel plus warfarin, and 60.8% dual antiplatelets. For cardiovascular outcome, adjusted hazard ratio with 95% confidence interval (HR) for triple therapy was 0.86 (0.70–1.07) for 0–90 days and 0.78 (0.58–1.05) for 91–365 days. A HR of 2.16 (1.48–3.13) and 1.61 (0.98–2.66) during 0–90 and 91–365 days, respectively, was observed for major bleeds. For aspirin plus warfarin, HR 0.82 (0.54–1.26) and 0.62 (0.48–0.79) was observed for cardiovascular outcome and 1.30 (0.60–2.85) and 1.01 (0.63–1.62) for major bleeds during 0–90 and 91–365 days, respectively. For clopidogrel plus warfarin, HR of 0.90 (0.68–1.19) and 0.68 (0.49–0.95) was observed for cardiovascular outcome and 1.28 (0.71–2.32) and 1.08 (0.57–2.04) for major bleeds during 0–90 and 91–365 days, respectively.
Compared to dual antiplatelets, aspirin or clopidogrel plus warfarin therapy was associated with similar 0–90 days and lower 91–365 days of risk of the cardiovascular outcome, without higher risk of major bleeds. Triple therapy was associated with non-significant lower risk of cardiovascular outcome and higher risk of major bleeds.
Introduction
Atrial fibrillation (AF) is an arrhythmia frequently encountered in patients hospitalized for acute myocardial infarction (MI), with a reported incidence up to 21%.1 Atrial fibrillation in the setting of MI is associated with increased risk of mortality and thrombo-embolic events.1
Dual antiplatelet therapy with aspirin and P2Y12 inhibitors is standard antithrombotic therapy for secondary prevention after MI and percutaneous coronary intervention (PCI).2 Treatment with oral anticoagulants is superior to antiplatelet therapy and reduces the incidence of ischaemic stroke in patients with AF.3 However, a clinical problem arises when patients with AF are hospitalized for MI and undergo PCI, as they then are in the need for secondary prophylaxis with antiplatelet agents, usually aspirin and P2Y12 inhibitors,2 as well as stroke prevention with oral anticoagulants.3
Today, guidelines from Europe recommend the use of triple therapy with aspirin, clopidogrel and an oral anticoagulant during an initial period after MI in patients with AF at increased risk for stroke.4,5 However, three randomized controlled trials have recently reported significantly higher bleeding risk when on triple therapy as compared to therapy with an oral anticoagulant in combination with a P2Y12 inhibitor.6–8 Nevertheless, no adequately powered randomized controlled trial has to date evaluated the efficacy of triple therapy. Also, meta-analyses show conflicting data regarding the efficacy of triple therapy as compared to dual antiplatelet therapy in regard to cardiovascular outcomes.9–11
In the present study of patients admitted to all coronary care units in Sweden, we compared commonly used antithrombotic strategies after MI and PCI in patients with AF. We sought to evaluate the effectiveness and safety of antithrombotic therapies in patients over time after discharge.
Methods
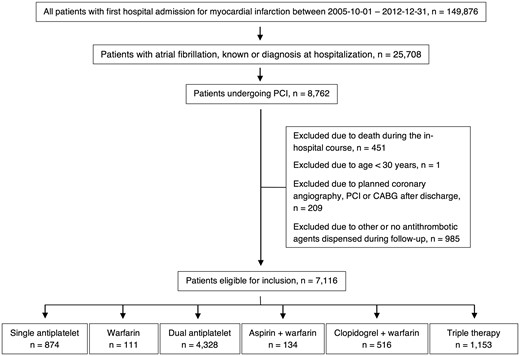
This is an observational multicentre study of all consecutive patients in Sweden with a first admission to any coronary care unit due to ST-segment elevation MI (STEMI) or non-ST-segment elevation MI (NSTEMI) between October 2005 and December 2012. The study included all patients who underwent in-hospital PCI due to their MI, and included only patients who had a previous diagnosis of AF or an electrocardiography finding of AF during the in-hospital course. Patients under 30 years and those not surviving the hospital course were excluded. As specific drug combinations were examined, patients dispensing none or other antithrombotic agents than those studied during follow-up were excluded (Figure 1, see Supplementary material online, Table S1).
Flow diagram illustrating the inclusion and exclusion of patients.
Data from the nationwide SWEDEHEART register were used to collect information about previous comorbidities, the in-hospital course, and discharge medication. SWEDEHEART includes all coronary care units in Sweden (n = 72), and patients are informed about their inclusion in the register and have the possibility to opt-out.12 Data about comorbidities were enriched by data linking SWEDEHEART with the National Patient Register using the unique 10-digit civic registration number of each citizen. The National Patient Register lists diagnoses for all hospital admissions and hospital associated open care visits in Sweden since 1987 and has been shown to have high validity13 (see Supplementary material online, Tables S2 and S3).
Information about medication at arrival, discharge, and during follow-up was obtained by data linking SWEDEHEART with the National Dispensed Drug Register. All prescribed drug purchases in Sweden, including oral anticoagulants and antiplatelet drugs, are reported to the register which includes data about Anatomical Therapeutic Chemical classification codes, dates, dosages, and quantities for every prescription that has been dispensed since July 2005. Medication at baseline was determined using data entered in SWEDEHEART at discharge and was cross-matched with information from the National Dispensed Drug Register. Only information about warfarin, aspirin, and clopidogrel was collected in the study, since novel antithrombotic agents were not routinely used in the setting of MI and AF in Sweden during the study period. For aspirin and clopidogrel, the purchased quantity was used to determine the duration of treatment. An interruption gap of 20 days between dispenses was allowed to identify detectable gaps in dispensing data. For warfarin, as dose varies over time, each purchase was estimated to last 3 months with an interruption gap of 3 months between dispenses being allowed as previously published.14 The risk of stroke was calculated using the CHA2DS2-VASc scores15 (see Supplementary material online, Table S4).
The predefined primary cardiovascular outcome was a composite of all-cause mortality, MI, or ischaemic stroke. Secondary outcomes included all-cause mortality, MI or coronary death, stent thrombosis, and ischaemic stroke. A safety outcome included all major bleeds requiring in-hospital attention, with separate analyses of intracranial and gastrointestinal bleeds. Complete 1-year outcome, with no loss in follow-up, was available for all patients after discharge for MI. Data regarding readmission due to MI, ischaemic stroke, and major bleeding events were based on International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes from the National Patient Register and the National Cause of Death Register. Data regarding readmission due to stent thrombosis were obtained from SWEDEHEART. Cause of death and death dates were obtained from the National Cause of Death Register (see Supplementary material online, Table S5).
The study was approved by the local ethics committee at Karolinska Institutet, and the data linkage was approved and performed by the Swedish National Board of Health and Welfare.
Statistics
Descriptive patient characteristics according to antithrombotic treatment at discharge are presented in a tabular format with percentages or as median with interquartile range (IQR). Crude event rates were calculated according to the number of events per 100 person-years. Simon–Makuch plots with a 0–90 and 91–365 days of landmark were plotted in order to illustrate the effect of antithrombotic medication during follow-up as a time-dependent factor. The association between antithrombotic medication during follow-up and outcome was assessed using time-varying Cox regression analysis allowing patients to switch antithrombotic regimen during follow-up. Two models were analysed, one unadjusted model and one model adjusting for potential confounding factors including new-onset AF during the in-hospital course, and factors in the CHA2DS2-VASc scoring system15; congestive heart failure, hypertension, age, diabetes mellitus, stroke, vascular disease, and gender. Continuous variables entered the Cox regression model using cubic splines to allow for the violation of the linearity assumption. Because of data enrichment to SWEDEHEART from the National Patient Register, no data were missing when adjusting for differences in baseline characteristics. Landmark analysis in combination with a time-varying Cox regression model was calculated to estimate the risk of the cardiovascular and safety outcome during an early (0–90 days) and late (91–365 days) period. Time until first event was evaluated for all outcomes during the respective landmark periods. No other censoring scheme was applied for the all-cause mortality outcome and the composite cardiovascular outcome. For all other outcomes, patients were also censored for mortality. In the landmark analysis for the late period between 91 and 365 days, patients were also censored if they died during the first 90 days or stopped taking any of the antithrombotic medications studied.
Three sensitivity analyses were calculated. The first sensitivity analysis was an intention-to-treat analysis using only data about antithrombotic medication at discharge. In a second sensitivity analysis, patients were censored at the time of change of drug treatment. In a final sensitivity model, the duration of warfarin treatment was calculated based on information about the purchased quantity and the average warfarin weekly dosage of 28.91 mg warfarin in patients with AF in Sweden according to the Swedish National Quality Registry for Atrial Fibrillation and Anticoagulation (AuriculA).16
All P-values are two-sided and stated as significant if less than 0.05. Statistical data analyses were performed using R-statistics 2.15.9.
Results
Patient characteristics
The study consisted of 7116 consecutive patients with first admission for MI undergoing PCI and with a history of AF or an in-hospital diagnosis of AF. Baseline characteristics in relation to antithrombotic therapy at discharge are presented in Table 1. The median age in the population was 76 years. During the index-hospitalization, 39.0% had a STEMI and 61.0% a NSTEMI. Revascularization with PCI with one or more stents was performed in 89.8% of the patients while 10.2% underwent PCI without stenting. Further patient and periprocedural characteristics are presented in Table 1.
Baseline table
| Characteristic, n (%) . | Single antiplatelet . | Warfarin . | Dual antiplatelets . | Aspirin + warfarin . | Clopidogrel + warfarin . | Triple therapy . |
|---|---|---|---|---|---|---|
| 874 (12.3) . | 111 (1.6) . | 4328 (60.8) . | 134 (1.9) . | 516 (7.3) . | 153 (16.2) . | |
| Age, years, median (IQR) | 78.0 (71.0–83.0) | 76.0 (71.0–80.0) | 76.0 (68.0–81.0) | 77.0 (70.0–81.0) | 76.0 (70.0–80.0) | 74.0 (68.0–79.0) |
| Gender, women | 297 (34.0) | 44 (39.6) | 1319 (30.5) | 44 (32.8) | 125 (24.2) | 276 (23.9) |
| Disease history at admission | ||||||
| Diabetes mellitus | 166 (19.0) | 28 (25.2) | 954 (22.0) | 43 (32.1) | 128 (24.8) | 531 (46.1) |
| Hypertension | 727 (83.2) | 96 (86.5) | 3244 (75.0) | 100 (74.6) | 404 (78.3) | 931 (80.7) |
| Ischaemic stroke | 118 (13.5) | 23 (20.7) | 427 (9.9) | 17 (12.7) | 74 (14.3) | 130 (11.3) |
| Chronic obstructive pulmonary disease | 106 (12.1) | 10 (9.0) | 331 (7.6) | 12 (9.0) | 37 (7.2) | 83 (7.2) |
| Congestive heart failure | 303 (34.7) | 49 (44.1) | 998 (23.1) | 36 (26.9) | 158 (30.6) | 314 (27.2) |
| Myocardial infarction | 480 (54.9) | 39 (35.1) | 1647 (38.1) | 55 (41.0) | 201 (39.0) | 353 (30.6) |
| Renal disease | 60 (6.9) | 1 (0.9) | 194 (4.5) | 4 (3.0) | 16 (3.1) | 37 (3.2) |
| Liver disease | 5 (0.6) | 0 (0.0) | 22 (0.5) | 1 (0.7) | 4 (0.8) | 8 (0.7) |
| Bleeding | 126 (14.4) | 12 (10.8) | 374 (8.6) | 11 (8.2) | 51 (9.9) | 95 (8.2) |
| Arrival antithrombotic medication | ||||||
| Aspirin | 579 (76.4) | 19 (32.2) | 2380 (55.0) | 48 (35.8) | 106 (41.2) | 300 (26.0) |
| Clopidogrel | 98 (13.8) | 3 (8.3) | 380 (8.8) | 2 (10.5) | 27 (5.2) | 62 (5.4) |
| Warfarin | 133 (49.8) | 67 (60.4) | 469 (36.5) | 67 (50.0) | 330 (64.0) | 719 (62.4) |
| In-hospital course | ||||||
| NSTEMI | 533 (61.0) | 51 (45.9) | 2577 (59.5) | 64 (47.8) | 334 (64.7) | 782 (67.8) |
| STEMI | 341 (39.0) | 60 (54.1) | 1751 (40.5) | 70 (52.2) | 182 (35.3) | 371 (32.2) |
| New-onset AF | 260 (29.7) | 38 (34.2) | 1619 (37.4) | 54 (40.3) | 162 (31.4) | 402 (34.9) |
| PCI characteristics | ||||||
| Vascular access approach | ||||||
| Radial-artery approach | 295 (33.8) | 45 (40.5) | 1725 (39.9) | 58 (43.3) | 232 (45.0) | 628 (54.5) |
| Femoral-artery approach | 520 (59.5) | 61 (55.0) | 2377 (54.9) | 67 (50.0) | 256 (49.6) | 444 (38.5) |
| Radial and femoral-artery approach | 58 (6.6) | 5 (4.5) | 219 (5.1) | 9 (6.7) | 28 (5.4) | 78 (6.8) |
| Other vascular approach | 1 (0.1) | 0 (0.0) | 7 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.3) |
| Finding | ||||||
| Normal/atheroma | 7 (0.8) | 2 (1.8) | 48 (1.1) | 1 (0.7) | 5 (1.0) | 7 (0.6) |
| One-vessel disease | 308 (35.2) | 52 (46.8) | 1676 (38.7) | 64 (47.8) | 218 (42.2) | 505 (43.8) |
| Two-vessel disease | 228 (26.1) | 26 (23.4) | 1234 (28.5) | 31 (23.1) | 124 (24.0) | 334 (29.0) |
| Three-vessel disease | 235 (26.9) | 23 (20.7) | 1047 (24.2) | 21 (15.7) | 133 (25.8) | 222 (19.3) |
| Left main coronary artery disease | 96 (11.0) | 8 (7.2) | 314 (7.3) | 15 (11.2) | 36 (7.0) | 82 (7.1) |
| Inconclusive | 0 (0.0) | 0 (0.0) | 9 (0.2) | 2 (1.5) | 0 (0.0) | 3 (0.3) |
| Number of stents | ||||||
| No stents | 152 (17.4) | 60 (54.1) | 311 (7.2) | 99 (73.9) | 36 (7.0) | 69 (6.0) |
| 1 stent | 448 (51.3) | 24 (21.6) | 2485 (57.4) | 24 (17.9) | 300 (58.1) | 678 (58.8) |
| 2 stents | 183 (20.9) | 15 (13.5) | 1026 (23.7) | 6 (4.5) | 113 (21.9) | 272 (23.6) |
| ≥3 stents | 91 (10.4) | 12 (10.8) | 506 (11.7) | 5 (3.7) | 67 (13.0) | 134 (11.6) |
| Bare metal stent group, n = 6389a | 500 (69.3) | 44 (86.3) | 2752 (68.5) | 28 (80.0) | 348 (72.5) | 774 (71.4) |
| Drug eluting stent group, n = 6389a | 222 (30.7) | 7 (13.7) | 1265 (31.5) | 7 (20.0) | 132 (27.5) | 310 (28.6) |
| CHA2DS2-VASc score | ||||||
| CHA2DS2-VASc score = 1 | 8 (0.9) | 2 (1.8) | 187 (4.3) | 5 (3.7) | 9 (1.7) | 30 (2.6) |
| CHA2DS2-VASc score ≥ 2 | 866 (99.1) | 109 (98.2) | 4141 (95.7) | 129 (96.3) | 507 (98.3) | 1123 (97.4) |
| Characteristic, n (%) . | Single antiplatelet . | Warfarin . | Dual antiplatelets . | Aspirin + warfarin . | Clopidogrel + warfarin . | Triple therapy . |
|---|---|---|---|---|---|---|
| 874 (12.3) . | 111 (1.6) . | 4328 (60.8) . | 134 (1.9) . | 516 (7.3) . | 153 (16.2) . | |
| Age, years, median (IQR) | 78.0 (71.0–83.0) | 76.0 (71.0–80.0) | 76.0 (68.0–81.0) | 77.0 (70.0–81.0) | 76.0 (70.0–80.0) | 74.0 (68.0–79.0) |
| Gender, women | 297 (34.0) | 44 (39.6) | 1319 (30.5) | 44 (32.8) | 125 (24.2) | 276 (23.9) |
| Disease history at admission | ||||||
| Diabetes mellitus | 166 (19.0) | 28 (25.2) | 954 (22.0) | 43 (32.1) | 128 (24.8) | 531 (46.1) |
| Hypertension | 727 (83.2) | 96 (86.5) | 3244 (75.0) | 100 (74.6) | 404 (78.3) | 931 (80.7) |
| Ischaemic stroke | 118 (13.5) | 23 (20.7) | 427 (9.9) | 17 (12.7) | 74 (14.3) | 130 (11.3) |
| Chronic obstructive pulmonary disease | 106 (12.1) | 10 (9.0) | 331 (7.6) | 12 (9.0) | 37 (7.2) | 83 (7.2) |
| Congestive heart failure | 303 (34.7) | 49 (44.1) | 998 (23.1) | 36 (26.9) | 158 (30.6) | 314 (27.2) |
| Myocardial infarction | 480 (54.9) | 39 (35.1) | 1647 (38.1) | 55 (41.0) | 201 (39.0) | 353 (30.6) |
| Renal disease | 60 (6.9) | 1 (0.9) | 194 (4.5) | 4 (3.0) | 16 (3.1) | 37 (3.2) |
| Liver disease | 5 (0.6) | 0 (0.0) | 22 (0.5) | 1 (0.7) | 4 (0.8) | 8 (0.7) |
| Bleeding | 126 (14.4) | 12 (10.8) | 374 (8.6) | 11 (8.2) | 51 (9.9) | 95 (8.2) |
| Arrival antithrombotic medication | ||||||
| Aspirin | 579 (76.4) | 19 (32.2) | 2380 (55.0) | 48 (35.8) | 106 (41.2) | 300 (26.0) |
| Clopidogrel | 98 (13.8) | 3 (8.3) | 380 (8.8) | 2 (10.5) | 27 (5.2) | 62 (5.4) |
| Warfarin | 133 (49.8) | 67 (60.4) | 469 (36.5) | 67 (50.0) | 330 (64.0) | 719 (62.4) |
| In-hospital course | ||||||
| NSTEMI | 533 (61.0) | 51 (45.9) | 2577 (59.5) | 64 (47.8) | 334 (64.7) | 782 (67.8) |
| STEMI | 341 (39.0) | 60 (54.1) | 1751 (40.5) | 70 (52.2) | 182 (35.3) | 371 (32.2) |
| New-onset AF | 260 (29.7) | 38 (34.2) | 1619 (37.4) | 54 (40.3) | 162 (31.4) | 402 (34.9) |
| PCI characteristics | ||||||
| Vascular access approach | ||||||
| Radial-artery approach | 295 (33.8) | 45 (40.5) | 1725 (39.9) | 58 (43.3) | 232 (45.0) | 628 (54.5) |
| Femoral-artery approach | 520 (59.5) | 61 (55.0) | 2377 (54.9) | 67 (50.0) | 256 (49.6) | 444 (38.5) |
| Radial and femoral-artery approach | 58 (6.6) | 5 (4.5) | 219 (5.1) | 9 (6.7) | 28 (5.4) | 78 (6.8) |
| Other vascular approach | 1 (0.1) | 0 (0.0) | 7 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.3) |
| Finding | ||||||
| Normal/atheroma | 7 (0.8) | 2 (1.8) | 48 (1.1) | 1 (0.7) | 5 (1.0) | 7 (0.6) |
| One-vessel disease | 308 (35.2) | 52 (46.8) | 1676 (38.7) | 64 (47.8) | 218 (42.2) | 505 (43.8) |
| Two-vessel disease | 228 (26.1) | 26 (23.4) | 1234 (28.5) | 31 (23.1) | 124 (24.0) | 334 (29.0) |
| Three-vessel disease | 235 (26.9) | 23 (20.7) | 1047 (24.2) | 21 (15.7) | 133 (25.8) | 222 (19.3) |
| Left main coronary artery disease | 96 (11.0) | 8 (7.2) | 314 (7.3) | 15 (11.2) | 36 (7.0) | 82 (7.1) |
| Inconclusive | 0 (0.0) | 0 (0.0) | 9 (0.2) | 2 (1.5) | 0 (0.0) | 3 (0.3) |
| Number of stents | ||||||
| No stents | 152 (17.4) | 60 (54.1) | 311 (7.2) | 99 (73.9) | 36 (7.0) | 69 (6.0) |
| 1 stent | 448 (51.3) | 24 (21.6) | 2485 (57.4) | 24 (17.9) | 300 (58.1) | 678 (58.8) |
| 2 stents | 183 (20.9) | 15 (13.5) | 1026 (23.7) | 6 (4.5) | 113 (21.9) | 272 (23.6) |
| ≥3 stents | 91 (10.4) | 12 (10.8) | 506 (11.7) | 5 (3.7) | 67 (13.0) | 134 (11.6) |
| Bare metal stent group, n = 6389a | 500 (69.3) | 44 (86.3) | 2752 (68.5) | 28 (80.0) | 348 (72.5) | 774 (71.4) |
| Drug eluting stent group, n = 6389a | 222 (30.7) | 7 (13.7) | 1265 (31.5) | 7 (20.0) | 132 (27.5) | 310 (28.6) |
| CHA2DS2-VASc score | ||||||
| CHA2DS2-VASc score = 1 | 8 (0.9) | 2 (1.8) | 187 (4.3) | 5 (3.7) | 9 (1.7) | 30 (2.6) |
| CHA2DS2-VASc score ≥ 2 | 866 (99.1) | 109 (98.2) | 4141 (95.7) | 129 (96.3) | 507 (98.3) | 1123 (97.4) |
Demographic and baseline characteristics were reported using percentages for categorical variables, or with median and IQR for continuous variables (as noted).
In the bare metal stent group, only patients receiving bare metal stents were included. In the drug eluting stent group, patients receiving at least one drug eluting stent was included, regardless of if they also received a bare metal stent.
Baseline table
| Characteristic, n (%) . | Single antiplatelet . | Warfarin . | Dual antiplatelets . | Aspirin + warfarin . | Clopidogrel + warfarin . | Triple therapy . |
|---|---|---|---|---|---|---|
| 874 (12.3) . | 111 (1.6) . | 4328 (60.8) . | 134 (1.9) . | 516 (7.3) . | 153 (16.2) . | |
| Age, years, median (IQR) | 78.0 (71.0–83.0) | 76.0 (71.0–80.0) | 76.0 (68.0–81.0) | 77.0 (70.0–81.0) | 76.0 (70.0–80.0) | 74.0 (68.0–79.0) |
| Gender, women | 297 (34.0) | 44 (39.6) | 1319 (30.5) | 44 (32.8) | 125 (24.2) | 276 (23.9) |
| Disease history at admission | ||||||
| Diabetes mellitus | 166 (19.0) | 28 (25.2) | 954 (22.0) | 43 (32.1) | 128 (24.8) | 531 (46.1) |
| Hypertension | 727 (83.2) | 96 (86.5) | 3244 (75.0) | 100 (74.6) | 404 (78.3) | 931 (80.7) |
| Ischaemic stroke | 118 (13.5) | 23 (20.7) | 427 (9.9) | 17 (12.7) | 74 (14.3) | 130 (11.3) |
| Chronic obstructive pulmonary disease | 106 (12.1) | 10 (9.0) | 331 (7.6) | 12 (9.0) | 37 (7.2) | 83 (7.2) |
| Congestive heart failure | 303 (34.7) | 49 (44.1) | 998 (23.1) | 36 (26.9) | 158 (30.6) | 314 (27.2) |
| Myocardial infarction | 480 (54.9) | 39 (35.1) | 1647 (38.1) | 55 (41.0) | 201 (39.0) | 353 (30.6) |
| Renal disease | 60 (6.9) | 1 (0.9) | 194 (4.5) | 4 (3.0) | 16 (3.1) | 37 (3.2) |
| Liver disease | 5 (0.6) | 0 (0.0) | 22 (0.5) | 1 (0.7) | 4 (0.8) | 8 (0.7) |
| Bleeding | 126 (14.4) | 12 (10.8) | 374 (8.6) | 11 (8.2) | 51 (9.9) | 95 (8.2) |
| Arrival antithrombotic medication | ||||||
| Aspirin | 579 (76.4) | 19 (32.2) | 2380 (55.0) | 48 (35.8) | 106 (41.2) | 300 (26.0) |
| Clopidogrel | 98 (13.8) | 3 (8.3) | 380 (8.8) | 2 (10.5) | 27 (5.2) | 62 (5.4) |
| Warfarin | 133 (49.8) | 67 (60.4) | 469 (36.5) | 67 (50.0) | 330 (64.0) | 719 (62.4) |
| In-hospital course | ||||||
| NSTEMI | 533 (61.0) | 51 (45.9) | 2577 (59.5) | 64 (47.8) | 334 (64.7) | 782 (67.8) |
| STEMI | 341 (39.0) | 60 (54.1) | 1751 (40.5) | 70 (52.2) | 182 (35.3) | 371 (32.2) |
| New-onset AF | 260 (29.7) | 38 (34.2) | 1619 (37.4) | 54 (40.3) | 162 (31.4) | 402 (34.9) |
| PCI characteristics | ||||||
| Vascular access approach | ||||||
| Radial-artery approach | 295 (33.8) | 45 (40.5) | 1725 (39.9) | 58 (43.3) | 232 (45.0) | 628 (54.5) |
| Femoral-artery approach | 520 (59.5) | 61 (55.0) | 2377 (54.9) | 67 (50.0) | 256 (49.6) | 444 (38.5) |
| Radial and femoral-artery approach | 58 (6.6) | 5 (4.5) | 219 (5.1) | 9 (6.7) | 28 (5.4) | 78 (6.8) |
| Other vascular approach | 1 (0.1) | 0 (0.0) | 7 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.3) |
| Finding | ||||||
| Normal/atheroma | 7 (0.8) | 2 (1.8) | 48 (1.1) | 1 (0.7) | 5 (1.0) | 7 (0.6) |
| One-vessel disease | 308 (35.2) | 52 (46.8) | 1676 (38.7) | 64 (47.8) | 218 (42.2) | 505 (43.8) |
| Two-vessel disease | 228 (26.1) | 26 (23.4) | 1234 (28.5) | 31 (23.1) | 124 (24.0) | 334 (29.0) |
| Three-vessel disease | 235 (26.9) | 23 (20.7) | 1047 (24.2) | 21 (15.7) | 133 (25.8) | 222 (19.3) |
| Left main coronary artery disease | 96 (11.0) | 8 (7.2) | 314 (7.3) | 15 (11.2) | 36 (7.0) | 82 (7.1) |
| Inconclusive | 0 (0.0) | 0 (0.0) | 9 (0.2) | 2 (1.5) | 0 (0.0) | 3 (0.3) |
| Number of stents | ||||||
| No stents | 152 (17.4) | 60 (54.1) | 311 (7.2) | 99 (73.9) | 36 (7.0) | 69 (6.0) |
| 1 stent | 448 (51.3) | 24 (21.6) | 2485 (57.4) | 24 (17.9) | 300 (58.1) | 678 (58.8) |
| 2 stents | 183 (20.9) | 15 (13.5) | 1026 (23.7) | 6 (4.5) | 113 (21.9) | 272 (23.6) |
| ≥3 stents | 91 (10.4) | 12 (10.8) | 506 (11.7) | 5 (3.7) | 67 (13.0) | 134 (11.6) |
| Bare metal stent group, n = 6389a | 500 (69.3) | 44 (86.3) | 2752 (68.5) | 28 (80.0) | 348 (72.5) | 774 (71.4) |
| Drug eluting stent group, n = 6389a | 222 (30.7) | 7 (13.7) | 1265 (31.5) | 7 (20.0) | 132 (27.5) | 310 (28.6) |
| CHA2DS2-VASc score | ||||||
| CHA2DS2-VASc score = 1 | 8 (0.9) | 2 (1.8) | 187 (4.3) | 5 (3.7) | 9 (1.7) | 30 (2.6) |
| CHA2DS2-VASc score ≥ 2 | 866 (99.1) | 109 (98.2) | 4141 (95.7) | 129 (96.3) | 507 (98.3) | 1123 (97.4) |
| Characteristic, n (%) . | Single antiplatelet . | Warfarin . | Dual antiplatelets . | Aspirin + warfarin . | Clopidogrel + warfarin . | Triple therapy . |
|---|---|---|---|---|---|---|
| 874 (12.3) . | 111 (1.6) . | 4328 (60.8) . | 134 (1.9) . | 516 (7.3) . | 153 (16.2) . | |
| Age, years, median (IQR) | 78.0 (71.0–83.0) | 76.0 (71.0–80.0) | 76.0 (68.0–81.0) | 77.0 (70.0–81.0) | 76.0 (70.0–80.0) | 74.0 (68.0–79.0) |
| Gender, women | 297 (34.0) | 44 (39.6) | 1319 (30.5) | 44 (32.8) | 125 (24.2) | 276 (23.9) |
| Disease history at admission | ||||||
| Diabetes mellitus | 166 (19.0) | 28 (25.2) | 954 (22.0) | 43 (32.1) | 128 (24.8) | 531 (46.1) |
| Hypertension | 727 (83.2) | 96 (86.5) | 3244 (75.0) | 100 (74.6) | 404 (78.3) | 931 (80.7) |
| Ischaemic stroke | 118 (13.5) | 23 (20.7) | 427 (9.9) | 17 (12.7) | 74 (14.3) | 130 (11.3) |
| Chronic obstructive pulmonary disease | 106 (12.1) | 10 (9.0) | 331 (7.6) | 12 (9.0) | 37 (7.2) | 83 (7.2) |
| Congestive heart failure | 303 (34.7) | 49 (44.1) | 998 (23.1) | 36 (26.9) | 158 (30.6) | 314 (27.2) |
| Myocardial infarction | 480 (54.9) | 39 (35.1) | 1647 (38.1) | 55 (41.0) | 201 (39.0) | 353 (30.6) |
| Renal disease | 60 (6.9) | 1 (0.9) | 194 (4.5) | 4 (3.0) | 16 (3.1) | 37 (3.2) |
| Liver disease | 5 (0.6) | 0 (0.0) | 22 (0.5) | 1 (0.7) | 4 (0.8) | 8 (0.7) |
| Bleeding | 126 (14.4) | 12 (10.8) | 374 (8.6) | 11 (8.2) | 51 (9.9) | 95 (8.2) |
| Arrival antithrombotic medication | ||||||
| Aspirin | 579 (76.4) | 19 (32.2) | 2380 (55.0) | 48 (35.8) | 106 (41.2) | 300 (26.0) |
| Clopidogrel | 98 (13.8) | 3 (8.3) | 380 (8.8) | 2 (10.5) | 27 (5.2) | 62 (5.4) |
| Warfarin | 133 (49.8) | 67 (60.4) | 469 (36.5) | 67 (50.0) | 330 (64.0) | 719 (62.4) |
| In-hospital course | ||||||
| NSTEMI | 533 (61.0) | 51 (45.9) | 2577 (59.5) | 64 (47.8) | 334 (64.7) | 782 (67.8) |
| STEMI | 341 (39.0) | 60 (54.1) | 1751 (40.5) | 70 (52.2) | 182 (35.3) | 371 (32.2) |
| New-onset AF | 260 (29.7) | 38 (34.2) | 1619 (37.4) | 54 (40.3) | 162 (31.4) | 402 (34.9) |
| PCI characteristics | ||||||
| Vascular access approach | ||||||
| Radial-artery approach | 295 (33.8) | 45 (40.5) | 1725 (39.9) | 58 (43.3) | 232 (45.0) | 628 (54.5) |
| Femoral-artery approach | 520 (59.5) | 61 (55.0) | 2377 (54.9) | 67 (50.0) | 256 (49.6) | 444 (38.5) |
| Radial and femoral-artery approach | 58 (6.6) | 5 (4.5) | 219 (5.1) | 9 (6.7) | 28 (5.4) | 78 (6.8) |
| Other vascular approach | 1 (0.1) | 0 (0.0) | 7 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.3) |
| Finding | ||||||
| Normal/atheroma | 7 (0.8) | 2 (1.8) | 48 (1.1) | 1 (0.7) | 5 (1.0) | 7 (0.6) |
| One-vessel disease | 308 (35.2) | 52 (46.8) | 1676 (38.7) | 64 (47.8) | 218 (42.2) | 505 (43.8) |
| Two-vessel disease | 228 (26.1) | 26 (23.4) | 1234 (28.5) | 31 (23.1) | 124 (24.0) | 334 (29.0) |
| Three-vessel disease | 235 (26.9) | 23 (20.7) | 1047 (24.2) | 21 (15.7) | 133 (25.8) | 222 (19.3) |
| Left main coronary artery disease | 96 (11.0) | 8 (7.2) | 314 (7.3) | 15 (11.2) | 36 (7.0) | 82 (7.1) |
| Inconclusive | 0 (0.0) | 0 (0.0) | 9 (0.2) | 2 (1.5) | 0 (0.0) | 3 (0.3) |
| Number of stents | ||||||
| No stents | 152 (17.4) | 60 (54.1) | 311 (7.2) | 99 (73.9) | 36 (7.0) | 69 (6.0) |
| 1 stent | 448 (51.3) | 24 (21.6) | 2485 (57.4) | 24 (17.9) | 300 (58.1) | 678 (58.8) |
| 2 stents | 183 (20.9) | 15 (13.5) | 1026 (23.7) | 6 (4.5) | 113 (21.9) | 272 (23.6) |
| ≥3 stents | 91 (10.4) | 12 (10.8) | 506 (11.7) | 5 (3.7) | 67 (13.0) | 134 (11.6) |
| Bare metal stent group, n = 6389a | 500 (69.3) | 44 (86.3) | 2752 (68.5) | 28 (80.0) | 348 (72.5) | 774 (71.4) |
| Drug eluting stent group, n = 6389a | 222 (30.7) | 7 (13.7) | 1265 (31.5) | 7 (20.0) | 132 (27.5) | 310 (28.6) |
| CHA2DS2-VASc score | ||||||
| CHA2DS2-VASc score = 1 | 8 (0.9) | 2 (1.8) | 187 (4.3) | 5 (3.7) | 9 (1.7) | 30 (2.6) |
| CHA2DS2-VASc score ≥ 2 | 866 (99.1) | 109 (98.2) | 4141 (95.7) | 129 (96.3) | 507 (98.3) | 1123 (97.4) |
Demographic and baseline characteristics were reported using percentages for categorical variables, or with median and IQR for continuous variables (as noted).
In the bare metal stent group, only patients receiving bare metal stents were included. In the drug eluting stent group, patients receiving at least one drug eluting stent was included, regardless of if they also received a bare metal stent.
Risk scores and medication at discharge
The CHA2DS2-VASc score was ≥2 in 96.6% of the patients. At discharge, 12.3% of the patients received single antiplatelet therapy, 1.6% warfarin monotherapy, 60.8% dual antiplatelet therapy (aspirin and clopidogrel), 1.9% aspirin plus warfarin, 7.3% clopidogrel plus warfarin and 16.2% triple therapy; in total, 26.9% received warfarin or a combination that included warfarin at discharge.
Cardiovascular outcomes
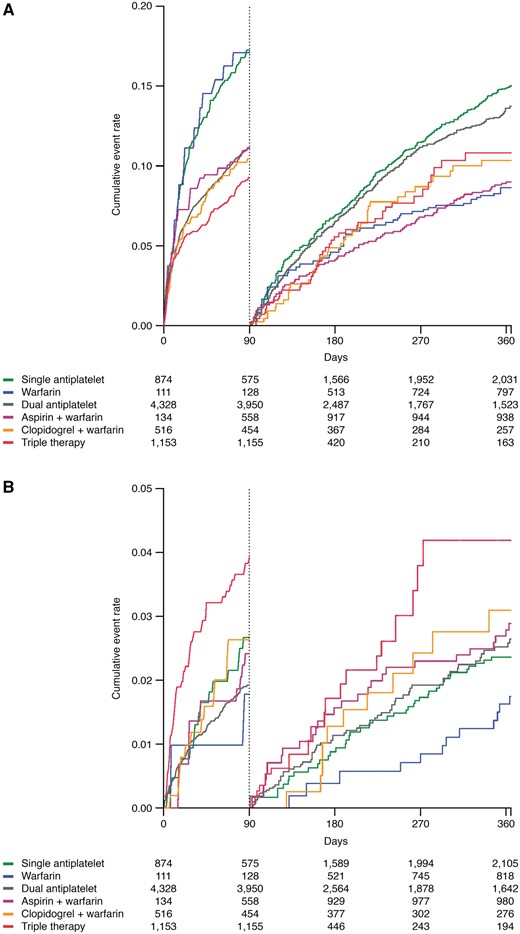
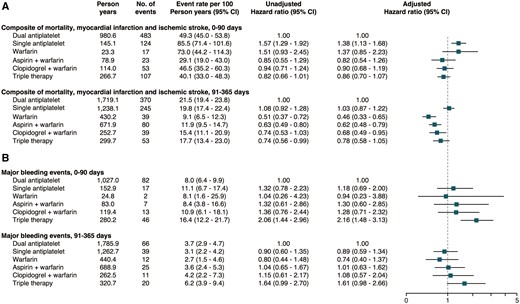
Landmark 0–90-day and 91–365-day analyses of the composite cardiovascular outcome of all-cause mortality, MI or ischaemic stroke are presented in Figures 2 and 3. As compared with dual antiplatelet therapy, the adjusted hazard ratio with 95% confidence interval (HR) for the composite cardiovascular outcome during 0–90 days was 1.38 (1.13–1.68) for single antiplatelet therapy, 1.37 (0.85–2.23) for warfarin monotherapy, 0.82 (0.54–1.26) for aspirin plus warfarin, 0.90 (0.68–1.19) for clopidogrel plus warfarin and 0.86 (0.70–1.07) for triple therapy, respectively. In the landmark analysis from 91 to 365 days, compared to dual antiplatelet therapy, the HR was 0.46 (0.33–0.65) for warfarin monotherapy, 0.62 (0.48–0.79) for aspirin plus warfarin, 0.68 (0.49–0.95) for clopidogrel plus warfarin and 0.78 (0.58–1.05) for triple therapy.
Simon–Makuch plots with a 0–90 and 91–365 days of landmark illustrating (A) cardiovascular outcome; all-cause mortality, myocardial infarction, or ischaemic stroke and (B) major bleeds requiring in-hospital attention according to use of antithrombotic therapy over time.
Forest plot depicting (A) cardiovascular outcome; all-cause mortality, myocardial infarction, or ischaemic stroke and (B) major bleeds requiring in-hospital attention according to use of antithrombotic therapy over time.
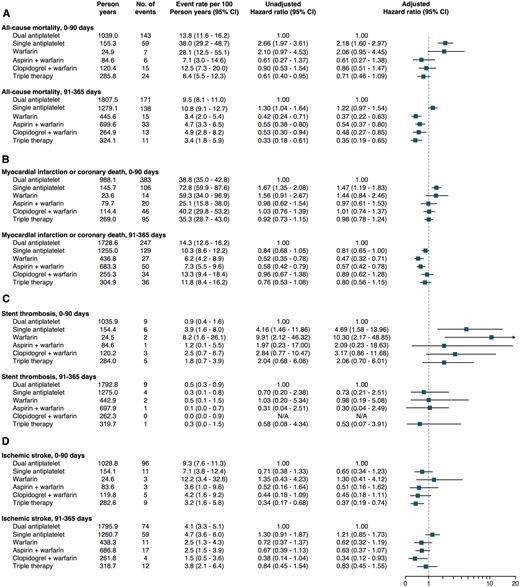
All-cause mortality, MI or coronary death, stent thrombosis and ischaemic stroke outcomes are presented in Figure 4. In the landmark analysis for all-cause mortality between 0 and 90 days and compared to dual antiplatelet therapy, a HR of 2.18 (1.60–2.97) and 2.06 (0.95–4.45) was seen for single antiplatelet therapy and warfarin monotherapy, respectively. Aspirin plus warfarin, clopidogrel plus warfarin and triple therapy did not significantly differ from dual antiplatelet therapy between 0 and 90 days. For single antiplatelet therapy, a similar but non-significant result was observed in the landmark analysis from 91 to 365 days. Between 91 and 365 days, warfarin monotherapy, aspirin plus warfarin, clopidogrel plus warfarin and triple therapy were all associated with lower HR of all-cause mortality than dual antiplatelet therapy.
Forest plot depicting (A) all-cause mortality, (B) myocardial infarction or coronary death, (C) stent thrombosis, and (D) ischaemic stroke according to use of antithrombotic therapy over time.
In the landmark analysis from 0 to 90 days, single antiplatelet therapy compared to dual antiplatelet therapy was associated with higher HR of MI or coronary death, HR 1.47 (1.19–1.83). No other significant associations were seen during the first 90 days. In the 91–365-day landmark analysis, the HR for MI or coronary death was 0.47 (0.32–0.71) for warfarin monotherapy and 0.57 (0.42–0.78) for aspirin plus warfarin. No significant difference was seen for clopidogrel plus warfarin and triple therapy compared to dual antiplatelet therapy.
Only 43 events of stent thrombosis were recorded in the total population during a 1 year of follow-up. Single antiplatelet therapy and warfarin monotherapy were significantly associated with higher HR of stent thrombosis as compared to dual antiplatelet therapy in the 0–90 days of landmark analysis. No other significant associations were observed.
Compared to dual antiplatelet therapy, the 0–90 days of HR of ischaemic stroke was 0.51 (0.16–1.62) for aspirin plus warfarin and 0.45 (0.18–1.11) for clopidogrel plus warfarin. In a combined cohort of patients treated with single antiplatelet, i.e. aspirin or clopidogrel plus warfarin, the 0–90 days of HR of ischaemic stroke was 0.47 (0.23–0.97). For triple therapy, the 0–90 days of HR of ischaemic stroke was 0.37 (0.19–0.74). Single antiplatelet plus warfarin therapy was significantly associated with a lower HR of ischaemic stroke in the 91–365 days of landmark analysis, adjusted HR 0.54 (0.33–0.88). All other treatment strategies showed no significant differences when compared to dual antiplatelet therapy.
Major bleeds requiring in-hospital attention
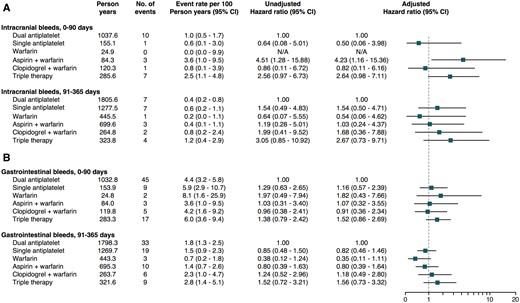
In patients undergoing PCI and treated with triple therapy, as compared with dual antiplatelet therapy, the adjusted 0–90 days of HR of major bleeds requiring in-hospital attention was 2.16 (1.48–3.13). In the landmark analysis between 91 and 365 days, triple therapy was associated with a borderline significant higher HR, 1.61 (0.98–2.66). No differences in major bleeds were seen across the other treatment strategies (Figures 2 and 3). Few events of intracranial bleeds were recorded during follow-up. As compared to dual antiplatelet therapy, only aspirin plus warfarin was associated with a higher risk of intracranial bleeds, HR 4.23 (1.16–15.36). Triple therapy showed a borderline higher risk of 0–90 day intracranial bleeds, HR 2.64 (0.98–7.11). All other treatment strategies were comparable to dual antiplatelet therapy in regard to intracranial bleeding events. No significant differences were seen across treatment strategies in regard to gastrointestinal bleeds requiring in-hospital attention (Figure 5).
Forest plot depicting (A) intracranial bleeds and (B) gastrointestinal bleeds requiring in-hospital attention according to use of antithrombotic therapy over time.
Sensitivity analyses
Three sensitivity analyses were conducted. In the intention-to-treat analysis, based only on antithrombotic medication at discharge, the results were similar to the findings for the landmark analysis between 0 and 90 days for the composite outcome (see Supplementary material online, Table S6). The second sensitivity analysis compared antithrombotic medication in patients until their first change in treatment regimen over time after which the patients were censored. The results in this analysis showed similar outcomes as in the landmark analysis between 0 and 90 days (see Supplementary material online, Table S7). The third sensitivity analyses used a different model for calculating the duration of warfarin treatment based on the average weekly dosage of 28.91 mg warfarin in patients with AF in Sweden. This analysis resulted in similar findings as seen in the main study design (see Supplementary material online, Table S8).
Discussion
In this large cohort of consecutive patients with MI and AF undergoing PCI in Sweden, we found that aspirin or clopidogrel plus warfarin was associated with a similar 0–90 days and a lower 91–365 days of risk of the composite of mortality, MI, or ischaemic stroke compared with dual antiplatelet therapy. Treatment with either aspirin or clopidogrel plus warfarin was not associated with increased risk of major bleeds compared to dual antiplatelet therapy. Triple therapy was associated with a similar risk of the composite of mortality, MI, or ischaemic stroke, but with an almost doubled risk of major bleeds requiring in-hospital attention than dual antiplatelet therapy. Notably, our study also illustrates that only 26.9% of the patients were prescribed oral anticoagulation with warfarin, with or without concomitant antiplatelet therapy at discharge.
Recent guidelines suggest the use of triple therapy during a period after coronary stenting in patients with MI and AF.4,5 However, these recommendations are mainly based on small single-centre or retrospective studies with inherent limitations.17–19 Consequently, optimal antithrombotic therapy for patients with AF post-MI is unknown. In the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial,2 dual antiplatelet therapy with aspirin and clopidogrel was superior to aspirin alone in patients with acute coronary syndromes. In patients with AF, the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE-W)3 indicated that oral anticoagulants was superior to aspirin plus clopidogrel at preventing stroke and systemic embolism, with a trend for reduction in MI. This is in line with older studies on warfarin after MI during the pre-PCI era, which showed that treatment with oral anticoagulants reduced the risk of mortality and re-infarction compared to placebo or aspirin.20,21
To date, no large randomized trial has examined the population of patients with AF and a strong indication for oral anticoagulants in the setting of MI. Our real-life data suggests that triple therapy, in the PCI treated patients after MI and with AF, significantly increases the risk of major bleeds, including intracranial bleeding events, with only a modest non-significant lower risk of cardiovascular outcome. However, a combination of either aspirin plus warfarin or clopidogrel plus warfarin does not seem to be associated with an increased risk of major bleeds, but with a lower risk of cardiovascular outcome than dual antiplatelet therapy.
The findings in our study are consistent with the What is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting (WOEST) trial6 in which patients undergoing PCI and taking oral anticoagulants, regardless of indication, were randomized to either triple therapy or dual therapy with an oral anticoagulant plus clopidogrel. This study was powered to show differences in the primary endpoint of any bleeding events within 1 year and showed that the use of an oral anticoagulants plus clopidogrel, as compared to triple therapy, reduced the risk of bleeding events with no increase in rate of thrombo-embolic events. However, the WOEST trial included only 573 patients of whom 69% had AF as the indication for oral anticoagulation, few patients in the WOEST trial underwent PCI due to acute coronary syndrome (27%) and patients on triple therapy received it continuous for 12 months. In addition, the WOEST trial only compared an oral anticoagulant plus clopidogrel vs. triple therapy, but did not study the combination of an oral anticoagulant plus aspirin, while the combinations of an oral anticoagulant with either aspirin or clopidogrel are reported in our study.
A observational study by Lamberts et al.22 reports the risk of bleeding and thrombo-embolic events in 12 165 patients with AF after MI or PCI. It showed that treatment with oral anticoagulants and clopidogrel, as compared with triple therapy, had similar bleed risk with no increase in all-cause mortality, MI/coronary death, and ischaemic stroke. Our results are largely consistent with this study. Compared to their study ours may offer some strengths as we have a more homogenous and rigorous inclusion criterion including only patients with MI and AF undergoing PCI rather than all AF patients hospitalized due to MI, regardless of if they undergo PCI or not, and/or undergo elective PCI. In a recent single-centre observational study by Choi et al.,23 the risk of cardiovascular events (cardiovascular death, MI, or stroke) and major bleeding events was studied in 711 patients with AF undergoing coronary stenting. In an intention-to-treat analysis, relative to dual antiplatelet therapy, triple therapy was associated with similar risk of cardiac events, but with a higher risk of bleeding events. These findings are similar to our results, however, based only on an intention-to-treat analysis. In our nationwide study, we were able to follow antithrombotic prescription over time and were able to adjust for changes in antithrombotic drug treatment combinations after discharge.
The risk of stent thrombosis is a serious complication and of great concern after PCI where studies have shown a beneficial effect with dual antiplatelet therapy compared to single antiplatelet therapy.2 Our study confirms that the risk of stent thrombosis might be increased in patients treated with single antiplatelet therapy than dual antiplatelets during the first 90 days after discharge. However, no significant differences were seen for single antiplatelet plus warfarin therapy or triple therapy vs. dual antiplatelet therapy in regard to stent thrombosis. Furthermore, our real-life data suggests that the rate of stent thrombosis is low and that omitting oral anticoagulation in favour of dual antiplatelet therapy due to concerns of stent thrombosis might result in net negative outcome. Also, of great concern in our real-life study is that only 26.9% were prescribed oral anticoagulation treatment, with or without concomitant antiplatelet therapy, at discharge even though the vast majority of patients had an indication for oral anticoagulation therapy according to the CHA2DS2-VASc score.15
During recent years, new and potent antiplatelet agents such as prasugrel and ticagrelor have been evaluated with beneficial effects in patients with MI, with sparse data in patients with AF.24 Moreover, non-vitamin K antagonist oral anticoagulants (NOAC) have shown to be at least as effective and safe as warfarin in patients with AF.25 Still, there is limited information about the benefits and risks with combinations of NOAC and antiplatelet agents, with a meta-analysis of randomized controlled trials in patients with a recent acute coronary syndrome showing an substantially increased risk of bleeding events with NOAC added to single or dual antiplatelets, but only a modest reduction in cardiovascular events.26 Two recently published trials, Open-Label, Randomized, Controlled, Multicentre Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI)7 and Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (RE-DUAL PCI)8 have reported that triple therapy with aspirin, a P2Y12 inhibitor and warfarin was associated with significantly higher bleeding risk compared to treatment strategies including either rivaroxaban or dabigatran in combination with a P2Y12 inhibitor. The thrombo-embolic event rates seemed similar in all treatment arms, although both these trials were not powered to evaluate efficacy outcomes. Another ongoing trial, the AUGUSTUS trial (NCT02415400), will also provide information on the efficacy and safety of apixaban in different combinations with antiplatelet agents post-PCI in patients with AF. However, no study on triple vs. dual combinations of oral anticoagulants and antiplatelets have prospectively evaluated if aspirin or a P2Y12 inhibitor should be the preferred single antiplatelet drug in combination with an oral anticoagulant.
Limitations
This is an observational cohort study with inherent limitations affecting the conclusions that can be drawn. First, patients were not randomized to the different treatment arms and we had no information regarding provider rationale for treatment choices. Although we were able to adjust for important confounders, the information about comorbidity was limited to the diseases that were registered in SWEDEHEART or the National Patient Register, with ample room for confounding by indication. As such, there might be other unmeasured confounders affecting the choice for antithrombotic strategies that were not adjusted for, such as individual preferences, practice patterns, and as an example the use of bare metal stents in about 70% of the patient population. However, any selection bias is likely to affect the estimates conservatively as healthier patients probably more often received triple therapy. We also had limited information about patient adherence to specific treatment regimens, except drug prescriptions being dispensed during follow-up according to the National Dispensed Drug Register, which could possibly lead to both an underestimation or overestimation of cardiovascular and bleeding risk. Also, our study lacks data on anticoagulation control. Nevertheless, our patient cohort represent a real-life population, and it has been previously shown that anticoagulation control in Sweden generally is very good with average time in therapeutic range >75%.27 Also, the bleeding outcome was restricted to hospitalizations for bleeding events or death related to bleeding events. Thus, the risk of bleeding is probably underestimated. Finally, we did not have brain-imaging data. Thus, some strokes reported as ischaemic may actually have been haemorrhagic.
Conclusions
In patients with MI and AF undergoing PCI, we found a substantial underutilization of oral anticoagulation with approximately one-quarter of the patients receiving warfarin, despite 97% of them having a strong indication for such therapy based on CHA2DS2-VASc score ≥ 2. Compared to dual antiplatelet therapy, treatment with warfarin plus a single antiplatelet agent was associated with a non-significant lower cardiovascular risk during the first 90 days and a significant lower risk between 91 and 365 days, without a higher risk of major bleeding events. Triple therapy was associated with a non-significant lower cardiovascular risk and a substantially higher risk of major bleeds.
Supplementary material
Supplementary material is available at European Heart Journal—Cardiovascular Pharmacotherapy online.
Acknowledgements
The authors extend their gratitude to the people involved in the SWEDEHEART registry, including health care personnel and registry administrators, for making this study possible.
Funding
This work was supported by a grant from the Swedish Foundation for Strategic Research, Stockholm, Sweden (Grant Number KF10-0024 to Tailoring of treatment in all comers with AMI).
Conflict of interest: G.B., none. L.F., grants, consultancy and lectures fees from Bayer, Bristol-Myers-Squibb, Pfizer, Sanofi and St Jude Medical.
D.E., speaker’s fees from AstraZeneca.
S.J., institutional research grant, honoraria and consultant/advisory board fee from AstraZeneca; institutional research grant and consultant/advisory board fee from Medtronic; institutional research grants and honoraria from The Medicines Company; consultant/advisory board fees from Janssen, Bayer.
T.J., lectures and consultant fees from AstraZeneca, Aspen and MSD.
B.S., none.
L.W., institutional research grant, consultancy and lecture fees, travel support and honoraria from GlaxoSmithKline; institutional research grants, consultancy and lecture fees and travel support from AstraZeneca, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim; institutional research grant from Merck & Co., Roche; consultancy fees from Abbott; holds two patents involving GDF-15.
J.O., consultant and lecture fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Sanofi.