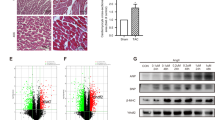
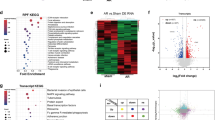
Abstract
Epigenetic reprogramming is a critical process of pathological gene induction during cardiac hypertrophy and remodeling, but the underlying regulatory mechanisms remain to be elucidated. Here we identified a heart-enriched long noncoding (lnc)RNA, named cardiac-hypertrophy-associated epigenetic regulator (Chaer), which is necessary for the development of cardiac hypertrophy. Mechanistically, Chaer directly interacts with the catalytic subunit of polycomb repressor complex 2 (PRC2). This interaction, which is mediated by a 66-mer motif in Chaer, interferes with PRC2 targeting to genomic loci, thereby inhibiting histone H3 lysine 27 methylation at the promoter regions of genes involved in cardiac hypertrophy. The interaction between Chaer and PRC2 is transiently induced after hormone or stress stimulation in a process involving mammalian target of rapamycin complex 1, and this interaction is a prerequisite for epigenetic reprogramming and induction of genes involved in hypertrophy. Inhibition of Chaer expression in the heart before, but not after, the onset of pressure overload substantially attenuates cardiac hypertrophy and dysfunction. Our study reveals that stress-induced pathological gene activation in the heart requires a previously uncharacterized lncRNA-dependent epigenetic checkpoint.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Sala, V. et al. Signaling to cardiac hypertrophy: insights from human and mouse RASopathies. Mol. Med. 18, 938–947 (2012).
Kuwahara, K., Nishikimi, T. & Nakao, K. Transcriptional regulation of the fetal cardiac gene program. J. Pharmacol. Sci. 119, 198–203 (2012).
Wang, Y. Mitogen-activated protein kinases in heart development and diseases. Circulation 116, 1413–1423 (2007).
Maron, B.J. & Maron, M.S. Hypertrophic cardiomyopathy. Lancet 381, 242–255 (2013).
Joh, R.I., Palmieri, C.M., Hill, I.T. & Motamedi, M. Regulation of histone methylation by noncoding RNAs. Biochim. Biophys. Acta 1839, 1385–1394 (2014).
Gould, A. Functions of mammalian Polycomb group and trithorax group–related genes. Curr. Opin. Genet. Dev. 7, 488–494 (1997).
Kaneda, R. et al. Genome-wide histone methylation profile for heart failure. Genes Cells 14, 69–77 (2009).
Angrisano, T. et al. Epigenetic switch at Atp2a2 and Myh7 gene promoters in pressure-overload-induced heart failure. PLoS One 9, e106024 (2014).
Papait, R. et al. Genome-wide analysis of histone marks identifying an epigenetic signature of promoters and enhancers underlying cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 110, 20164–20169 (2013).
Ishii, N. et al. Identification of a novel noncoding RNA, MIAT, that confers risk of myocardial infarction. J. Hum. Genet. 51, 1087–1099 (2006).
Friedrichs, F. et al. HBEGF, SRA1 and IK: three co-segregating genes as determinants of cardiomyopathy. Genome Res. 19, 395–403 (2009).
Burd, C.E. et al. Expression of linear and novel circular forms of an INK4/ARF-associated noncoding RNA correlates with atherosclerosis risk. PLoS Genet. 6, e1001233 (2010).
Kumarswamy, R. et al. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 114, 1569–1575 (2014).
Wang, K. et al. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 114, 1377–1388 (2014).
Jiang, F., Zhou, X. & Huang, J. Long noncoding RNA–ROR mediates the reprogramming in cardiac hypertrophy. PLoS One 11, e0152767 (2016).
Viereck, J. et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci. Transl. Med. 8, 326ra22 (2016).
Liu, L. et al. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc. Res. 111, 56–65 (2016).
Peters, T. et al. Long noncoding RNA Malat-1 is dispensable during pressure-overload-induced cardiac remodeling and failure in mice. PLoS One 11, e0150236 (2016).
Matkovich, S.J., Edwards, J.R., Grossenheider, T.C., de Guzman Strong, C. & Dorn, G.W., II. Epigenetic coordination of embryonic heart transcription by dynamically regulated long noncoding RNAs. Proc. Natl. Acad. Sci. USA 111, 12264–12269 (2014).
Ounzain, S. et al. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long noncoding RNAs. Eur. Heart J. 36, 353–68a (2015).
Grote, P. et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell 24, 206–214 (2013).
Lee, J.H. et al. Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts. Circ. Res. 109, 1332–1341 (2011).
Kurian, L. et al. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation 131, 1278–1290 (2015).
Wang, Z. & Wang, Y. Dawn of the Epi-LncRNAs: new path from Myheart. Circ. Res. 116, 235–236 (2015).
Li, L. et al. Targeted disruption of Hotair leads to homeotic transformation and gene de-repression. Cell Rep. 5, 3–12 (2013).
Klattenhoff, C.A. et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152, 570–583 (2013).
Wang, K.C. et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011).
Liu, Z. et al. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21WAF1/CIP1 expression. PLoS One 8, e77293 (2013).
Li, F. et al. Characterization of sucrose transporter alleles and their association with seed-yield-related traits in Brassica napus L. BMC Plant Biol. 11, 168 (2011).
Han, P. et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514, 102–106 (2014).
Kallen, A.N. et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 52, 101–112 (2013).
Monnier, P. et al. H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. Proc. Natl. Acad. Sci. USA 110, 20693–20698 (2013).
Finn, R.D. et al. Pfam: the protein families database. Nucleic Acids Res. 42, D222–D230 (2014).
Chu, C., Qu, K., Zhong, F.L., Artandi, S.E. & Chang, H.Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA–chromatin interactions. Mol. Cell 44, 667–678 (2011).
Leppek, K. & Stoecklin, G. An optimized streptavidin-binding RNA aptamer for purification of ribonucleoprotein complexes identifies novel ARE-binding proteins. Nucleic Acids Res. 42, e13 (2014).
Wu, L., Murat, P., Matak-Vinkovic, D., Murrell, A. & Balasubramanian, S. Binding interactions between long noncoding RNA HOTAIR and PRC2 proteins. Biochemistry 52, 9519–9527 (2013).
Kang, S., Chemaly, E.R., Hajjar, R.J. & Lebeche, D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase–mammalian target of rapamycin (AMPK–mTOR) and c-Jun N-terminal kinase–insulin receptor substrate 1 (JNK–IRS1) pathways. J. Biol. Chem. 286, 18465–18473 (2011).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Kim, D.H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Sarbassov, D.D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Li, Q., Wang, P., Ye, K. & Cai, H. Central role of SIAH inhibition in DCC-dependent cardioprotection provoked by netrin-1–NO. Proc. Natl. Acad. Sci. USA 112, 899–904 (2015).
Cabili, M.N. et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011).
Delgado-Olguín, P. et al. Epigenetic repression of cardiac progenitor gene expression by EZH2 is required for postnatal cardiac homeostasis. Nat. Genet. 44, 343–347 (2012).
Yang, T. et al. MicroRNA-214 provokes cardiac hypertrophy via repression of EZH2. Biochem. Biophys. Res. Commun. 436, 578–584 (2013).
Shi, L. et al. Histone demethylase JMJD2B coordinates H3K4 and H3K9 methylation and promotes hormonally responsive breast carcinogenesis. Proc. Natl. Acad. Sci. USA 108, 7541–7546 (2011).
Agger, K. et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449, 731–734 (2007).
Song, X. et al. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am. J. Physiol. Cell Physiol. 299, C1256–C1266 (2010).
Liu, X., Wang, X., Bi, Y., Bu, P. & Zhang, M. The histone demethylase PHF8 represses cardiac hypertrophy upon pressure overload. Exp. Cell Res. 335, 123–134 (2015).
Xu, N. et al. The alteration of protein prenylation induces cardiomyocyte hypertrophy through Rheb–mTORC1 signaling and leads to chronic heart failure. J. Pathol. 235, 672–685 (2015).
Li, Y. et al. AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch. Biochem. Biophys. 558, 79–86 (2014).
Shende, P. et al. Cardiac mTOR complex 2 preserves ventricular function in pressure-overload hypertrophy. Cardiovasc. Res. 109, 103–114 (2016).
Shende, P. et al. Cardiac Raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression and causes heart failure in mice. Circulation 123, 1073–1082 (2011).
Kaneko, S. et al. Phosphorylation of the PRC2 component EZH2 is cell-cycle-regulated and upregulates its binding to ncRNA. Genes Dev. 24, 2615–2620 (2010).
Marketou, M.E., Parthenakis, F. & Vardas, P.E. Pathological left ventricular hypertrophy and stem cells: current evidence and new perspectives. Stem Cells Int. 2016, 5720758 (2016).
National Research Council. Guide for the Care and Use of Laboratory Animals 8th edn. (The National Academies Press, 2011).
MacLellan, W.R., Xiao, G., Abdellatif, M. & Schneider, M.D. A novel Rb- and p300-binding protein inhibits transactivation by MyoD. Mol. Cell. Biol. 20, 8903–8915 (2000).
van Laake, L.W. et al. Reporter-based isolation of induced pluripotent stem cell– and embryonic stem cell–derived cardiac progenitors reveals limited gene expression variance. Circ. Res. 107, 340–347 (2010).
Ubil, E. et al. Mesenchymal–endothelial transition contributes to cardiac neovascularization. Nature 514, 585–590 (2014).
Zhou, Y.Y. et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am. J. Physiol. Heart Circ. Physiol. 279, H429–H436 (2000).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR–Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Komurov, K., Dursun, S., Erdin, S. & Ram, P.T. NetWalker: a contextual network analysis tool for functional genomics. BMC Genomics 13, 282 (2012).
Lu, G. et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Invest. 119, 1678–1687 (2009).
Nekrasov, M., Wild, B. & Müller, J. Nucleosome-binding and histone methyltransferase activity of Drosophila PRC2. EMBO Rep. 6, 348–353 (2005).
Baroni, T.E., Chittur, S.V., George, A.D. & Tenenbaum, S.A. Advances in RIP-chip analysis: RNA-binding protein immunoprecipitation–microarray profiling. Methods Mol. Biol. 419, 93–108 (2008).
Leppek, K. & Stoecklin, G. An optimized streptavidin-binding RNA aptamer for purification of ribonucleoprotein complexes identifies novel ARE-binding proteins. Nucleic Acids Res. 42, e13 (2014).
Sdek, P. et al. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. J. Cell Biol. 194, 407–423 (2011).
Young, M.D. et al. ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic Acids Res. 39, 7415–7427 (2011).
Streicher, J.M., Kamei, K., Ishikawa, T.O., Herschman, H. & Wang, Y. Compensatory hypertrophy induced by ventricular-cardiomyocyte-specific COX-2 expression in mice. J. Mol. Cell. Cardiol. 49, 88–94 (2010).
Mitchell-Jordan, S.A. et al. Loss of Bmx nonreceptor tyrosine kinase prevents pressure-overload-induced cardiac hypertrophy. Circ. Res. 103, 1359–1362 (2008).
Jiang, D.S. et al. Interferon regulatory factor 7 functions as a novel negative regulator of pathological cardiac hypertrophy. Hypertension 63, 713–722 (2014).
Acknowledgements
This work was supported in part by the US National Institute of Health (NIH) (grant no. HL070079 (Y.W.), HL103205 (Y.W.), HL108186 (Y.W.), HL110667 (Y.W.), HL115238 (T.M.V.), R01HG006264 (X.X.) and U01HG007013 (X.X.)), the University of California at Los Angeles CTSI–Cardiovascular Pilot Team research grant UL1TR000124 (Y.W., X.X. and T.M.V.), the National Science Fund for Distinguished Young Scholars (grant no. 81425005; H.L.), the Key Project of the National Natural Science Foundation (grant no. 81330005 and 81630011; both to H.L.), the National Science and Technology Support Project (grant no. 2013YQ030923-05, 2014BAI02B01 and 2015BAI08B01; all to H.L.) and an American Heart Association Western States Affiliate Postdoctoral Fellowship (15POST24970034; Z.W.). C.G. was a recipient of the UCLA Eli and Edythe Broad Center Predoctoral Fellowship in Stem Cell Science. H.W. was supported by the China Scholarship Council (award no. 201406280042) and was a recipient of a Jennifer S. Buchwald Graduate Fellowship in Physiology. A.C. was supported by NIH predoctoral training grant T90DE022734. The authors wish to acknowledge outstanding technical support from H. Pu and Core support from the UCLA Cardiovascular Research Laboratories and the Division of Molecular Medicine at UCLA.
Author information
Authors and Affiliations
Contributions
Z.W. designed all the experiments with input from H.-T.Y., H.L. and Y.W., performed the majority of the experiments and drafted the manuscript; Y.W. designed and supervised the study; H.L. designed and supervised the in vivo experiments; X.-J.Z., Y.-X.J., P.Z. and K.-Q.D. performed the northern blot, ChIP, FISH, immunoblotting and PV loop assays for Chaer-KO mice with input from H.L.; K.-Q.D., J.G. and H.L. developed the Chaer-knockout mice and performed some of the in vivo experiments; X.W. and G.L. participated in the tagged-RNA pulldown assay and RIP assays; I.C., C.G. and H.W. participated in the lncRNA screening and cloning experiments; T.Y. participated in the histology assay; A.C., X.X. and T.M.V. participated in the transcriptome analysis; S.R. performed the TAC surgery; Y.S.A. and D.S. provided the human iPSC-derived cardiomyocytes; S.L. and A.D. provided the primary cardiac fibroblasts and myocytes from mouse heart.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5 and Supplementary Figures 1–12 (PDF 5893 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Zhang, XJ., Ji, YX. et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med 22, 1131–1139 (2016). https://doi.org/10.1038/nm.4179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4179
This article is cited by
-
Circular RNA-circPan3 attenuates cardiac hypertrophy via miR-320-3p/HSP20 axis
Cellular & Molecular Biology Letters (2024)
-
Correlation between large rearrangements and patient phenotypes in NF1 deletion syndrome: an update and review
BMC Medical Genomics (2024)
-
Astragaloside IV derivative HHQ16 ameliorates infarction-induced hypertrophy and heart failure through degradation of lncRNA4012/9456
Signal Transduction and Targeted Therapy (2023)
-
Non-coding RNAs in human health and disease: potential function as biomarkers and therapeutic targets
Functional & Integrative Genomics (2023)
-
LOC102549726/miR-760-3p network is involved in the progression of ISO-induced pathological cardiomyocyte hypertrophy via endoplasmic reticulum stress
Journal of Molecular Histology (2023)