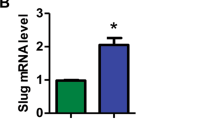
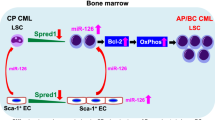
Abstract
Leukemias and other cancers possess self-renewing stem cells that help to maintain the cancer1,2. Cancer stem cell eradication is thought to be crucial for successful anticancer therapy. Using an acute myeloid leukemia (AML) model induced by the leukemia-associated monocytic leukemia zinc finger (MOZ)-TIF2 fusion protein, we show here that AML can be cured by the ablation of leukemia stem cells. The MOZ fusion proteins MOZ-TIF2 and MOZ-CBP interacted with the transcription factor PU.1 to stimulate the expression of macrophage colony–stimulating factor receptor (CSF1R, also known as M-CSFR, c-FMS or CD115). Studies using PU.1-deficient mice showed that PU.1 is essential for the ability of MOZ-TIF2 to establish and maintain AML stem cells. Cells expressing high amounts of CSF1R (CSF1Rhigh cells), but not those expressing low amounts of CSF1R (CSF1Rlow cells), showed potent leukemia-initiating activity. Using transgenic mice expressing a drug-inducible suicide gene controlled by the CSF1R promoter, we cured AML by ablation of CSF1Rhigh cells. Moreover, induction of AML was suppressed in CSF1R-deficient mice and CSF1R inhibitors slowed the progression of MOZ-TIF2–induced leukemia. Thus, in this subtype of AML, leukemia stem cells are contained within the CSF1Rhigh cell population, and we suggest that targeting of PU.1-mediated upregulation of CSF1R expression might be a useful therapeutic approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bonnet, D. & Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737 (1997).
Reya, T., Morrison, S.J., Clarke, M.F. & Weissman, I.L. Stem cells, cancer and cancer stem cells. Nature 414, 105–111 (2001).
Borrow, J. et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat. Genet. 14, 33–41 (1996).
Katsumoto, T., Yoshida, N. & Kitabayashi, I. Roles of the histone acetyltransferase monocytic leukemia zinc finger protein in normal and malignant hematopoiesis. Cancer Sci. 99, 1523–1527 (2008).
Katsumoto, T. et al. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 20, 1321–1330 (2006).
Thomas, T. et al. Monocytic leukemia zinc finger protein is essential for the development of long-term reconstituting hematopoietic stem cells. Genes Dev. 20, 1175–1186 (2006).
Huntly, B.J. et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 6, 587–596 (2004).
Deguchi, K. et al. MOZ-TIF2–induced acute myeloid leukemia requires the MOZ nucleosome binding motif and TIF2-mediated recruitment of CBP. Cancer Cell 3, 259–271 (2003).
Terui, K. et al. Two novel variants of MOZ-CBP fusion transcripts in spontaneously remitted infant leukemia with t(1;16;8)(p13;p13;p11), a new variant of t(8;16)(p11;p13). Haematologica 93, 1591–1593 (2008).
Burnett, S.H. et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukoc. Biol. 75, 612–623 (2004).
Kawagoe, H., Kandilci, A., Kranenburg, T.A. & Grosveld, G.C. Overexpression of N-Myc rapidly causes acute myeloid leukemia in mice. Cancer Res. 67, 10677–10685 (2007).
Dai, X.M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies and reproductive defects. Blood 99, 111–120 (2002).
Ohno, H. et al. A c-fms tyrosine kinase inhibitor, Ki20227, suppresses osteoclast differentiation and osteolytic bone destruction in a bone metastasis model. Mol. Cancer Ther. 5, 2634–2643 (2006).
Taylor, J.R., Brownlow, N., Domin, J. & Dibb, N.J. FMS receptor for M-CSF (CSF-1) is sensitive to the kinase inhibitor imatinib and mutation of Asp-802 to Val confers resistance. Oncogene 25, 147–151 (2006).
Dewar, A.L., Zannettino, A.C., Hughes, T.P. & Lyons, A.B. Inhibition of c-fms by imatinib: expanding the spectrum of treatment. Cell Cycle 4, 851–853 (2005).
Dewar, A.L. et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood 105, 3127–3132 (2005).
Zhang, D.E. et al. CCAAT enhancer-binding protein (C/EBP) and AML1 (CBF alpha2) synergistically activate the macrophage colony-stimulating factor receptor promoter. Mol. Cell. Biol. 16, 1231–1240 (1996).
Kitabayashi, I., Aikawa, Y., Nguyen, L.A., Yokoyama, A. & Ohki, M. Activation of AML1-mediated transcription by MOZ and inhibition by the MOZ-CBP fusion protein. EMBO J. 20, 7184–7196 (2001).
Hoogenkamp, M. et al. Early chromatin unfolding by RUNX1: a molecular explanation for differential requirements during specification versus maintenance of the hematopoietic gene expression program. Blood 114, 299–309 (2009).
Walsh, J.C. et al. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity 17, 665–676 (2002).
Kroon, E. et al. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J. 17, 3714–3725 (1998).
Jin, G. et al. Trib1 and Evi1 cooperate with Hoxa and Meis1 in myeloid leukemogenesis. Blood 109, 3998–4005 (2007).
Wang, C. et al. Expression of the CSF-1 gene in the blast cells of acute myeloblastic leukemia: association with reduced growth capacity. J. Cell. Physiol. 135, 133–138 (1988).
Rambaldi, A. et al. Expression of the macrophage colony–stimulating factor and c-fms genes in human acute myeloblastic leukemia cells. J. Clin. Invest. 81, 1030–1035 (1988).
Preisler, H.D., Kinniburgh, A.J., Wei-Dong, G. & Khan, S. Expression of the protooncogenes c-myc, c-fos and c-fms in acute myelocytic leukemia at diagnosis and in remission. Cancer Res. 47, 874–880 (1987).
Gisselbrecht, S. et al. Frequent c-fms activation by proviral insertion in mouse myeloblastic leukaemias. Nature 329, 259–261 (1987).
Heard, J.M., Roussel, M.F., Rettenmier, C.W. & Sherr, C.J. Multilineage hematopoietic disorders induced by transplantation of bone marrow cells expressing the v-fms oncogene. Cell 51, 663–673 (1987).
Gu, T.L. et al. A novel fusion of RBM6 to CSF1R in acute megakaryoblastic leukemia. Blood 110, 323–333 (2007).
Iwasaki, H. et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 106, 1590–1600 (2005).
Seibler, J. et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 31, e12 (2003).
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 (2000).
Zhang, D.E., Hetherington, C.J., Chen, H.M. & Tenen, D.G. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol. Cell. Biol. 14, 373–381 (1994).
Yoshida, H. et al. PML-retinoic acid receptor α inhibits PML IV enhancement of PU.1-induced C/EBPɛ expression in myeloid differentiation. Mol. Cell. Biol. 27, 5819–5834 (2007).
Acknowledgements
We would like to thank D.E. Zhang for the CSF1R promoter mutant lacking PU.1-binding sites, Y. Kamei and A. Iwama for MOZ-TIF2 cDNA, H. Ichikawa for N-MYC cDNA, T. Taya for SaOS2 cells (National Cancer Center Research Institute) and A. Kuchiba for help with statistical analyses. This work was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Health, Labor and Welfare and from the Japanese Ministry of Education, Culture, Sports, Science and Technology (I.K.), by the Program for Promotion of Fundamental Studies from the National Institute of Biomedical Innovation of Japan (I.K.), and by US National Institutes of Health grants R01-CA41456 (D.G.T.), CA32551 and 5P30-CA13330 (E.R.S.).
Author information
Authors and Affiliations
Contributions
Y.A., I.K., T.K. and M.S. conducted experiments in AML mice. Y.A., H. Shima and I.K. performed western blotting, immunoprecipitation, GST pull down, ChIP and reporter assays. P.Z. and D.G.T. conducted experiments in PU.1-deficient mice. E.R.S. designed and performed experiments in CSF1R-deficient mice. K.T. and E.I. analyzed expression of CSF1R in human AML cells. H. Singh designed and performed experiments in PUER cells. H.O. prepared Ki20227. I.K. and Y.A. analyzed data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 3848 kb)
Rights and permissions
About this article
Cite this article
Aikawa, Y., Katsumoto, T., Zhang, P. et al. PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2. Nat Med 16, 580–585 (2010). https://doi.org/10.1038/nm.2122
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2122
This article is cited by
-
Identification of female-enriched and disease-associated microglia (FDAMic) contributes to sexual dimorphism in late-onset Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Directional association test reveals high-quality putative cancer driver biomarkers including noncoding RNAs
BMC Medical Genomics (2019)
-
Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials
Signal Transduction and Targeted Therapy (2019)
-
A phase I trial of the aurora kinase inhibitor, ENMD-2076, in patients with relapsed or refractory acute myeloid leukemia or chronic myelomonocytic leukemia
Investigational New Drugs (2016)
-
PU.1 is essential for MLL leukemia partially via crosstalk with the MEIS/HOX pathway
Leukemia (2014)