Abstract
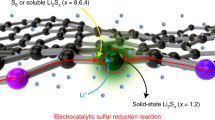
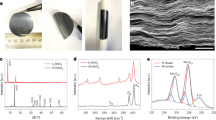
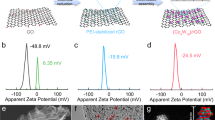
The sulfur reduction reaction (SRR) plays a central role in high-capacity lithium sulfur (Li-S) batteries. The SRR involves an intricate, 16-electron conversion process featuring multiple lithium polysulfide intermediates and reaction branches1,2,3. Establishing the complex reaction network is essential for rational tailoring of the SRR for improved Li-S batteries, but represents a daunting challenge4,5,6. Herein we systematically investigate the electrocatalytic SRR to decipher its network using the nitrogen, sulfur, dual-doped holey graphene framework as a model electrode to understand the role of electrocatalysts in acceleration of conversion kinetics. Combining cyclic voltammetry, in situ Raman spectroscopy and density functional theory calculations, we identify and directly profile the key intermediates (S8, Li2S8, Li2S6, Li2S4 and Li2S) at varying potentials and elucidate their conversion pathways. Li2S4 and Li2S6 were predominantly observed, in which Li2S4 represents the key electrochemical intermediate dictating the overall SRR kinetics. Li2S6, generated (consumed) through a comproportionation (disproportionation) reaction, does not directly participate in electrochemical reactions but significantly contributes to the polysulfide shuttling process. We found that the nitrogen, sulfur dual-doped holey graphene framework catalyst could help accelerate polysulfide conversion kinetics, leading to faster depletion of soluble lithium polysulfides at higher potential and hence mitigating the polysulfide shuttling effect and boosting output potential. These results highlight the electrocatalytic approach as a promising strategy for tackling the fundamental challenges regarding Li-S batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the main text, figures and Supplementary Information files. Source data are provided with this paper. All relevant data are available from the corresponding authors on request.
Code availability
The code used for simulated voltage-dependent concentrations and CV curves is available at https://github.com/lophocinalis/concentration_cv.
References
Mikhaylik, Y. V. & Akridge, J. R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 151, A1969–A1976 (2004).
Yin, Y. X., Xin, S., Guo, Y. G. & Wan, L. J. Lithium-sulfur batteries: electrochemistry, materials, and prospects. Angew. Chem. Int. Edn Engl. 52, 13186–13200 (2013).
Pang, Q., Liang, X., Kwok, C. Y. & Nazar, L. F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016).
Barchasz, C. et al. Lithium/sulfur cell discharge mechanism: an original approach for intermediate species identification. Anal. Chem. 84, 3973–3980 (2012).
Cuisinier, M. et al. Sulfur speciation in Li-S batteries determined by operando X-ray absorption spectroscopy. J. Phys. Chem. Lett. 4, 3227–3232 (2013).
Wild, M. et al. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 8, 3477–3494 (2015).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J. M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2011).
Manthiram, A., Fu, Y., Chung, S. H., Zu, C. & Su, Y. S. Rechargeable lithium-sulfur batteries. Chem. Rev. 114, 11751–11787 (2014).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Jayaprakash, N., Shen, J., Moganty, S. S., Corona, A. & Archer, L. A. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew. Chem. Int. Edn Engl. 50, 5904–5908 (2011).
Wang, N. et al. Thickness-independent scalable high-performance Li-S batteries with high areal sulfur loading via electron-enriched carbon framework. Nat. Commun. 12, 4519 (2021).
Wang, L. et al. A quantum-chemical study on the discharge reaction mechanism of lithium-sulfur batteries. J. Energy Chem. 22, 72–77 (2013).
Zou, Q. & Lu, Y. C. Solvent-dictated lithium sulfur redox reactions: an operando UV-vis spectroscopic study. J. Phys. Chem. Lett. 7, 1518–1525 (2016).
He, Q., Freiberg, A. T. S., Patel, M. U. M., Qian, S. & Gasteiger, H. A. Operando identification of liquid intermediates in lithium-sulfur batteries via transmission UV-vis spectroscopy. J. Electrochem. Soc. 167, 080508 (2020).
Zheng, D. et al. Investigation of the Li-S battery mechanism by real-time monitoring of the changes of sulfur and polysulfide species during the discharge and charge. ACS Appl. Mater. Interfaces 9, 4326–4332 (2017).
Peng, L. et al. A fundamental look at electrocatalytic sulfur reduction reaction. Nat. Catal. 3, 762–770 (2020).
Zhou, G. M., Paek, E., Hwang, G. S. & Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 6, 7760 (2015).
Yuan, Z. et al. Powering lithium-sulfur battery performance by propelling polysulfide redox at sulfiphilic hosts. Nano Lett. 16, 519–527 (2016).
Peng, H. J. et al. Enhanced electrochemical kinetics on conductive polar mediators for lithium-sulfur batteries. Angew. Chem. Int. Edn Engl. 55, 12990–12995 (2016).
Wang, L. et al. Li2S4 anchoring governs the catalytic sulfur reduction on defective SmMn2O5 in lithium-sulfur battery. Adv. Energy Mater. 12, 2200340 (2022).
Zhou, T. et al. Twinborn TiO2-TiN heterostructures enabling smooth trapping-diffusion-conversion of polysulfides towards ultralong life lithium-sulfur batteries. Energy Environ. Sci. 10, 1694–1703 (2017).
Zhou, J. et al. Deciphering the modulation essence of p bands in Co-based compounds on Li-S chemistry. Joule 2, 2681–2693 (2018).
Hua, W. et al. Selective catalysis remedies polysulfide shuttling in lithium‐sulfur batteries. Adv. Mater. 33, 2101006 (2021).
Zhou, G. et al. Theoretical calculation guided design of single-atom catalysts toward fast kinetic and long-life Li-S batteries. Nano Lett. 20, 1252–1261 (2019).
Wang, L. et al. Design rules of a sulfur redox electrocatalyst for lithium-sulfur batteries. Adv. Mater. 34, 2110279 (2022).
Lu, Y.-C., He, Q. & Gasteiger, H. A. Probing the lithium-sulfur redox reactions: a rotating-ring disk electrode study. J. Phys. Chem. C Nanomater. Interfaces 118, 5733–5741 (2014).
Conder, J. et al. Direct observation of lithium polysulfides in lithium-sulfur batteries using operando X-ray diffraction. Nat. Energy 2, 17069 (2017).
Wujcik, K. H. et al. Fingerprinting lithium-sulfur battery reaction products by X-ray absorption spectroscopy. J. Electrochem. Soc. 161, A1100–A1106 (2014).
Hou, T. Z. et al. Lithium bond chemistry in lithium-sulfur batteries. Angew. Chem. Int. Edn Engl. 129, 8290–8294 (2017).
Hagen, M. et al. In-situ Raman investigation of polysulfide formation in Li-S cells. J. Electrochem. Soc. 160, A1205–A1214 (2013).
Zhou, G. et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li-S batteries. Proc. Natl Acad. Sci. USA 114, 840–845 (2017).
Wujcik, K. H. et al. Characterization of polysulfide radicals present in an ether-based electrolyte of a lithium-sulfur battery during initial discharge using in situ X-ray absorption spectroscopy experiments and first‐principles calculations. Adv. Energy Mater. 5, 1500285 (2015).
Rajput, N. N. et al. Elucidating the solvation structure and dynamics of lithium polysulfides resulting from competitive salt and solvent interactions. Chem. Mater. 29, 3375–3379 (2017).
Assary, R. S., Curtiss, L. A. & Moore, J. S. Toward a molecular understanding of energetics in Li-S batteries using nonaqueous electrolytes: a high-level quantum chemical study. J. Phys. Chem. C Nanomater. Interfaces 118, 11545–11558 (2014).
Xu, Y., Sheng, K., Li, C. & Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4, 4324–4330 (2010).
Chen, J.-J. et al. Conductive lewis base matrix to recover the missing link of Li2S8 during the sulfur redox cycle in Li-S battery. Chem. Mater. 27, 2048–2055 (2015).
Zhu, X. et al. A highly stretchable cross-linked polyacrylamide hydrogel as an effective binder for silicon and sulfur electrodes toward durable lithium-ion storage. Adv. Funct. Mater. 28, 1705015 (2018).
Wu, H. L., Huff, L. A. & Gewirth, A. A. In situ Raman spectroscopy of sulfur speciation in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 7, 1709–1719 (2015).
Lei, T. et al. Inhibiting polysulfide shuttling with a graphene composite separator for highly robust lithium-sulfur batteries. Joule 2, 2091–2104 (2018).
Chen, W. et al. A new hydrophilic binder enabling strongly anchoring polysulfides for high-performance sulfur electrodes in lithium-sulfur battery. Adv. Energy Mater. 8, 1702889 (2018).
Hannauer, J. et al. The quest for polysulfides in lithium-sulfur battery electrolytes: an operando confocal Raman spectroscopy study. ChemPhysChem 16, 2709–2709 (2015).
Zhang, Z.-M., Chen, S. & Liang, Y.-Z. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 135, 1138–1146 (2010).
Zhu, W. et al. Investigation of the reaction mechanism of lithium sulfur batteries in different electrolyte systems by in situ Raman spectroscopy and in situ X-ray diffraction. Sustain. Energy Fuels 1, 737–747 (2017).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133 (1965).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993).
Sun, J., Ruzsinszky, A. & Perdew, J. P. Strongly constrained and appropriately normed semilocal density functional. Phys. Rev. Lett. 115, 036402 (2015).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Acknowledgements
This work is supported by the Center for Synthetic Control Across Length-scales for Advancing Rechargeables, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science Basic Energy Sciences programme under award no. DE-SC0019381.
Author information
Authors and Affiliations
Contributions
X.D. conceived the research. X.D. and R.L. designed the experimental research. P.S. and Z. Wei designed and performed density functional theory (DFT) calculations. R.L. performed experiments and conducted data analysis, with contributions from Z. Wei, L.P., L.Z., P.W., C.W., D.Z., H.L., Z. Wang, S.T., B.D., Y.H., P.S. and X.D. A.Z. and R.S. contributed to Raman data collection. R.L. and Z. Wei wrote the original draft. X.D. and P.S. revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Mahbub Islam, Quan-Hong Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs 1–17, Tables 1–16, Notes 1–11 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, R., Wei, Z., Peng, L. et al. Establishing reaction networks in the 16-electron sulfur reduction reaction. Nature 626, 98–104 (2024). https://doi.org/10.1038/s41586-023-06918-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06918-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.