Abstract
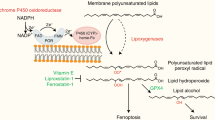
Ferroptosis, a form of regulated cell death that is driven by iron-dependent phospholipid peroxidation, has been implicated in multiple diseases, including cancer1,2,3, degenerative disorders4 and organ ischaemia–reperfusion injury (IRI)5,6. Here, using genome-wide CRISPR–Cas9 screening, we identified that the enzymes involved in distal cholesterol biosynthesis have pivotal yet opposing roles in regulating ferroptosis through dictating the level of 7-dehydrocholesterol (7-DHC)—an intermediate metabolite of distal cholesterol biosynthesis that is synthesized by sterol C5-desaturase (SC5D) and metabolized by 7-DHC reductase (DHCR7) for cholesterol synthesis. We found that the pathway components, including MSMO1, CYP51A1, EBP and SC5D, function as potential suppressors of ferroptosis, whereas DHCR7 functions as a pro-ferroptotic gene. Mechanistically, 7-DHC dictates ferroptosis surveillance by using the conjugated diene to exert its anti-phospholipid autoxidation function and shields plasma and mitochondria membranes from phospholipid autoxidation. Importantly, blocking the biosynthesis of endogenous 7-DHC by pharmacological targeting of EBP induces ferroptosis and inhibits tumour growth, whereas increasing the 7-DHC level by inhibiting DHCR7 effectively promotes cancer metastasis and attenuates the progression of kidney IRI, supporting a critical function of this axis in vivo. In conclusion, our data reveal a role of 7-DHC as a natural anti-ferroptotic metabolite and suggest that pharmacological manipulation of 7-DHC levels is a promising therapeutic strategy for cancer and IRI.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Oxi-lipidomic and lipidomic data are available online (https://data.mendeley.com/datasets/4prc7fy4zm/1). All other data are available in the Article and its Supplementary Information. The CRISPR screen data are provided in Supplementary Table 1. Gel source images are shown in Supplementary Fig. 2. STRING and GO analysis were conducted with the STRING database (https://string-db.org). CTRP data analysis was mined from https://portals.broadinstitute.org/ctrp.v2.1/. Fitness scores data were mined from https://score.depmap.sanger.ac.uk/. Source data are provided with this paper.
References
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Hangauer, M. J. et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017).
Chen, L., Hambright, W. S., Na, R. & Ran, Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 290, 28097–28106 (2015).
Linkermann, A. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl Acad. Sci. USA 111, 16836–16841 (2014).
Tonnus, W. et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tub controls iron homeostasis and ular necrosis during acute kidney injury. Nat. Commun. 12, 4402 (2021).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Do, Q., Zhang, R., Hooper, G. & Xi, L. Differential contributions of distinct free radical peroxidation mechanisms to the induction of ferroptosis. JACS Au. 3, 1100–1117 (2023).
Agmon, E., Solon, J., Bassereau, P. & Stockwell, B. R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 8, 5155 (2018).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Kraft, V. A. N. et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6, 41–53 (2020).
Soula, M. et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16, 1351–1360 (2020).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764 (2023).
Muller, C., Junker, J., Bracher, F. & Giera, M. A gas chromatography-mass spectrometry-based whole-cell screening assay for target identification in distal cholesterol biosynthesis. Nat. Protoc. 14, 2546–2570 (2019).
Yin, H., Xu, L. & Porter, N. A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111, 5944–5972 (2011).
Porter, N. A., Xu, L. & Pratt, D. A. Reactive sterol electrophiles: mechanisms of formation and reactions with proteins and amino acid nucleophiles. Chemistry 2, 390–417 (2020).
Xu, L., Davis, T. A. & Porter, N. A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131, 13037–13044 (2009).
Xu, L. et al. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 45, 923–929 (2012).
Pfeffer, B. A., Xu, L., Porter, N. A., Rao, S. R. & Fliesler, S. J. Differential cytotoxic effects of 7-dehydrocholesterol-derived oxysterols on cultured retina-derived cells: dependence on sterol structure, cell type, and density. Exp. Eye Res. 145, 297–316 (2016).
Porter, F. D. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 16, 535–541 (2008).
Schmitz, R. et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012).
Park, R. J. et al. A genome-wide CRISPR screen identifies a restricted set of HIV host dependency factors. Nat. Genet. 49, 193–203 (2017).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3, e02523 (2014).
Szklarczyk, D. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Rees, M. G. et al. Correlating chemical sensitivity and basal gene expression reveals mechanism of action. Nat. Chem. Biol. 12, 109–116 (2016).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Sun, X. et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63, 173–184 (2016).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966–E4975 (2016).
Genaro-Mattos, T. C. et al. Dichlorophenyl piperazines, including a recently-approved atypical antipsychotic, are potent inhibitors of DHCR7, the last enzyme in cholesterol biosynthesis. Toxicol. Appl. Pharmacol. 349, 21–28 (2018).
Garcia-Bermudez, J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122 (2019).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell 73, 354–363 (2019).
Gaschler, M. M. et al. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem. Biol. 13, 1013–1020 (2018).
Lamberson, C. R. et al. Propagation rate constants for the peroxidation of sterols on the biosynthetic pathway to cholesterol. Chem. Phys. Lipids 207, 51–58 (2017).
Shah, R., Farmer, L. A., Zilka, O., Van Kessel, A. T. M. & Pratt, D. A. Beyond DPPH: use of fluorescence-enabled inhibited autoxidation to predict oxidative cell death rescue. Cell Chem. Biol. 26, 1594–1607 (2019).
Yaoita, Y. et al. Sterol constituents from five edible mushrooms. Chem. Pharm. Bull. 46, 944–950 (1998).
Hong, X. et al. The lipogenic regulator SREBP2 induces transferrin in circulating melanoma cells and suppresses ferroptosis. Cancer Discov. 11, 678–695 (2021).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Chen, Y. et al. Quantitative profiling of protein carbonylations in ferroptosis by an aniline-derived probe. J. Am. Chem. Soc. 140, 4712–4720 (2018).
Theodoropoulos, P. C. et al. A medicinal chemistry-driven approach identified the sterol isomerase EBP as the molecular target of TASIN colorectal cancer toxins. J. Am. Chem. Soc. 142, 6128–6138 (2020).
Dwane, L. et al. Project Score database: a resource for investigating cancer cell dependencies and prioritizing therapeutic targets. Nucleic Acids Res. 49, D1365–D1372 (2021).
Ubellacker, J. M. et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118 (2020).
Rodgers, M. A., Saghatelian, A. & Yang, P. L. Identification of an overabundant cholesterol precursor in hepatitis B virus replicating cells by untargeted lipid metabolite profiling. J. Am. Chem. Soc. 131, 5030–5031 (2009).
Xiao, J. et al. Targeting 7-dehydrocholesterol reductase integrates cholesterol metabolism and IRF3 activation to eliminate infection. Immunity 52, 109–122 (2020).
Xu, L., Korade, Z. & Porter, N. A. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc. 132, 2222–2232 (2010).
Kaneski, C. R., Hanover, J. A. & Schueler Hoffman, U. H. Generation of an in vitro model for peripheral neuropathy in Fabry disease using CRISPR-Cas9 in the nociceptive dorsal root ganglion cell line 50B11. Mol. Genet. Metab. Rep. 31, 100871 (2022).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Kanarek, N. et al. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 559, 632–636 (2018).
Li, J. et al. Hiplot: a comprehensive and easy-to-use web service for boosting publication-ready biomedical data visualization. Brief. Bioinform. 23, bbac261 (2022).
Luu, W., Gelissen, I. C. & Brown, A. J. Manipulating cholesterol status within cells. Methods Mol. Biol. 1583, 41–52 (2017).
Anderson, R. H. et al. Sterols lower energetic barriers of membrane bending and fission necessary for efficient clathrin-mediated endocytosis. Cell Rep. 38, 110371 (2022).
Li, B. et al. Besting vitamin E: sidechain substitution is key to the reactivity of naphthyridinol antioxidants in lipid bilayers. J. Am. Chem. Soc. 135, 1394–1405 (2013).
Chen, W. W., Freinkman, E., Wang, T., Birsoy, K. & Sabatini, D. M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166, 1324–1337 (2016).
McDonald, J. G., Smith, D. D., Stiles, A. R. & Russell, D. W. A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J. Lipid Res. 53, 1399–1409 (2012).
Huang, H. et al. Plasma lipidomics identifies unique lipid signatures and potential biomarkers for patients with aortic dissection. Front. Cardiovasc. Med. 8, 757022 (2021).
Yin, H. et al. Identification of intact oxidation products of glycerophospholipids in vitro and in vivo using negative ion electrospray iontrap mass spectrometry. J. Mass Spectrom. 44, 672–680 (2009).
Van Coillie, S. et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat. Commun. 13, 1046 (2022).
Acknowledgements
We thank J. P. F. Angeli and C. Jiang for suggestions for the manuscript; C. Wang for providing guidance to the ABPP assay using m-APA; Z. Zhang for the structure analysis; Y. Li for help with providing platform of liposome production used in the FENIX assay; H. Wang for providing guidance for 7-DHC and cholesterol measurement; A. Qiu and W. Zhai for sharing information of the kidney IRI model; and H. Li for technical help. This study was supported by the National Key Research and Development Program of China (2022YFC3401500, 2021YFA1302200 and 2020YFA0803201), grants from the National Natural Science Foundation of China (31830053, 31920103007, 82341028, 82122056, 82073155, 82073153, 32030053, 22207084 and 32150710522), the Shanghai Sailing Program (22YF1433500), Shanghai Municipal Health Commission (20204Y0033), the Fundamental Research Funds for the Central Universities (22120220616 and PA2023000692), the fund from City University of Hong Kong (9380154, 7006046) and the Shanghai Rising-Star Program (23QA1407500).
Author information
Authors and Affiliations
Contributions
P.W. supervised and conceived the studies with support from Y.L., Q.R. and Q.D.; Y.L., Q.R., Q.D and P.W. designed experiments. Y.L., Q.R., Q.D. and J.J. performed most of the experiments and analysed the data. Y.L., Q.R. and Q.D. performed the CRISPR–Cas9 screen with help from L.W.; Q.R., Y.W. and J.J. conducted biochemical and cellular studies. Y.L. and Q.D. generated the majority of the CRISPR–Cas9 knockout cell lines and performed most of the animal studies with help from Y.W., Q.W. and H.T.; Q.D. and S.S. generated the plasmids and mutations. Z.Z. and H.Z. generated the platform of 7-DHC and other metabolite detection. X.C. and H.Y. performed the oxi-lipidomic analysis and analysed the data. H.W. and Q.Z. helped with 7-DHC detection. S.C. performed the lipidomics analysis. Z.S. performed bioinformatic analysis. Y.W., H.T. and J.W. performed immunohistochemistry experiments. L.Y. and C.W. synthesized the TASIN-30 compound. L.F. and H.Q. provided the platform and technical help. Y.L., Q.R., Q.D. Z.L., J.J., X.J. and P.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
X.J. is listed as an inventor on patents related to autophagy and cell death and holds equity in and consults for Exarta Therapeutics and Lime Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Andreas Linkermann, Sean Morrison, Ned Porter and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Identification of distal CB pathway including CYP51A1, MSMO1, EBP and SC5D as ferroptosis suppressors.
a, Integrative analysis of pooled CRISPR genetic screening using MAGeCK and identification of top candidate genes that were assigned with values generated from negative selection (anti-ferroptotic) by modified robust ranking aggregation (α-RRA) analysis. b, The normalized read counts of sgRNAs by CYP51A1, MSMO1, EBP and SC5D, generated by the MAGeCK-test module. n = 5 sgRNAs per gene. c, Schema of the cholesterol pathway. Top screen hits are shown as red (pro-ferroptotic) or blue (anti-ferroptotic). d, The protein-protein interaction network was constructed using the STRING database to identify the top screening hits. e, GO analysis (conducted by https://string-db.org) evaluates global functional category enrichment of gene lists and indicates pathway-level pattern enrichment of cholesterol synthesis from the 50 top scoring genes. FDR are p-values corrected for multiple testing using the Benjamini–Hochberg procedure. f, CTRP analysis (conducted with https://portals.broadinstitute.org/ctrp.v2.1/) shows that the expression levels of CYP51A1, MSMO1, EBP and SC5D, positively correlate with their resistance to ferroptosis induced by the compounds (i.e. RSL3, ML162, ML210 and Erastin) in cancer cells. Plotted values are z-scored Pearson’s correlation coefficients with minima and maxima of the distributions; line, median; box, 25th–75th percentile; whiskers, expansion of outlier compounds (red dots) according to the interquartile multiplier (as shown in right).
Extended Data Fig. 2 The distal cholesterol biosynthesis genes regulates ferroptosis.
a, Genotyping of subclones generated from HEK293T cells expressing sgRNAs targeting MSMO1, SC5D and EBP by Sanger sequencing. b, The protein level of CYP51A1 in CYP51A1 KO HEK293T cells was analysed by Immunoblotting. c, Cell viability of WT and CYP51A1, MSMO1, EBP, SC5D KO HEK293T cells treated with ML210 for 8–10 h. d, e, Cell viability of WT and CYP51A1, MSMO1, EBP, SC5D KO HEK293T cells treated with RSL3 for 6–8 h following pretreatment of DMSO, Fer-1 (1 μM), DFO (20 μM) and Idebenone (10 μM) (d) or Fer-1 (1 μM), Z-VAD-FMK (20 μM) and Nec-1s (20 μM) (e). f, The protein level of SC5D-FLAG in SC5D KO HEK293T cells expressing vector and SC5D-FLAG was analysed by Immunoblotting. g, The protein level of ACSL4 in SC5D KO HEK293T cells expressing sgRNAs targeting ACSL4 was analysed by Immunoblotting. h, The normalized read counts of sgRNAs by DHCR7, generated by the MAGeCK-test module. n = 5 sgRNAs. i, Integrative analysis of pooled CRISPR genetic screening using MAGeCK and identification of top candidate genes that were assigned with values generated from positive selection (pro-ferroptotic) by modified robust ranking aggregation (α-RRA) analysis. j, The protein level of DHCR7 in WT and DHCR7 KO HEK293T cells was detected by Immunoblotting. k, Cell viability of WT and DHCR7 KO HEK293T cells treated with ML210 for 8–10 h. l, The protein level of DHCR7-FLAG in DHCR7 KO HEK293T cells expressing vector and DHCR7-FLAG analysed by Immunoblotting. For b-g, j-l, data are representative of three independent experiments. Data are mean ± s.d. of n = 3 biological replicates.
Extended Data Fig. 3 Distal CB pathway regulates ferroptosis independent of cholesterol and known ferroptosis defence system.
a, Levels of cholesterol in WT and CYP51A1, MSMO1, EBP, SC5D, DHCR7 KO HEK293T cells. b, Relative concentration of cholesterol in FBS and LPDS measured by Amplex® Red Cholesterol Assay Kit. c, The concentrations of cholesterol in FBS and LPDS detected by LC-MS/MS. d, e, Cell viability in HEK293T (d) and HT1080 (e) cells treated with RSL3 for 6–8 h after pretreatment with or without cholesterol (25 μM) for 24 h in FBS- or LPDS-supplemented medium. n = 2 biological replicates. f, g, The protein levels of GPX4, FSP1, DHODH, ACSL4 and SLC7A11 in WT SC5D KO (f) and DHCR7 KO (g) HEK293T cells detected by immunoblotting. h, i, GSH/GSSG level measurement in WT, SC5D KO (c) and DHCR7 KO (d) HEK293T cells. j, k, FACS analysis of Fe2+ using FerroOrange probe by measuring MFI values in WT, SC5D KO (j) and DHCR7 KO (k) HEK293T cells. l, m, Levels of squalene (l) and CoQ10 (m) in WT, SC5D KO and DHCR7 KO HEK293T cells. Data are representative of two (a-c, l, m) and three (d-k) independent experiments. Data are mean ± s.d. of n = 3 biological replicates (a-c, h-m). Statistical analysis was performed using one-way ANOVA (a, h-m) or unpaired two-tailed t-tests (b and c); ****P < 0.0001; ns, not significant.
Extended Data Fig. 4 7-DHC suppresses ferroptosis.
a, The protein level of DHCR7 in SC5D KO HEK293T cells expressing sgRNAs targeting DHCR7 detected by Immunoblotting. b, g, The concentration of 7-DHC in WT and DHCR7 KO HEK293T cells after treatment of indicated concentrations of AY9944 (b) or cariprazine (g) for 48 h. c, h, Cell viability of WT, DHCR7 KO and SC5D KO HEK293T cells treated with RSL3 for 8 h after pretreatment of AY9944 (100 nM) (c) or cariprazine (100 nM) (h) for 48 h. d, Schema of cholesterol biosynthesis pathway. e, i, Cell viability of U937 and SNU-1 cells treated with RSL3 for 18 h after pretreatment of AY9944 (100 nM) (e) or cariprazine (100 nM) (i) for 48 h. f, j, Representative autoxidation inhibited by indicated concentration of AY9944 (f) and cariprazine (j). k, Levels of 7-DHC in HEK293T cells after treatment of indicated dose of ethanol-dissolved 7-DHC or MβCD-coated 7-DHC for 24 h. l, Cell viability of HEK293T cells treated with RSL3 for 8 h after pretreatment of ethanol-dissolved 7-DHC(25 μM) or MβCD-coated 7-DHC (25 μM) for 24 h. m, Cell viability of HEK293T cells treated with RSL3 for 8 h after pretreatment of MβCD-coated sterols (25 μM) for 24 h. Data are representative of at least two (a-c and e-m) independent experiments. Data are mean ± s.d. of n = 3 biological replicates (b, c, e, g-I and k-m). Statistical analysis was performed using two-way ANOVA (b, g and k); *P < 0.05, ****P < 0.0001, ns, not significant.
Extended Data Fig. 5 7-DHC is a general suppressor of ferroptosis.
a, Cell viability of various cancer cell lines treated with indicated concentrations of RSL3 for 6–8 h after pretreatment of 7-DHC (25 μM) for 24 h. For HuH-7, PLC/PRF/5 and SK-Hep1, n = 2 biological replicates and for 786-O, U2OS and HT1080, n = 3 biological replicates. b, Cell viability of U2OS and RCC4 treated with indicated concentrations of Erastin for 16 h after pretreatment of 7-DHC (25 μM) for 24 h. n = 2 biological replicates. c, d, Cell viability of various human cancer cell lines (human hepatocellular carcinoma cell lines HuH-7, PLC/PRF/5 and SK-Hep1; human kidney cancer cell lines Caki-1; human kidney cancer cell line 786-O; human osteosarcoma cell line U2OS; human fibrosarcoma cell line HT1080) treated with indicated concentrations of RSL3 for 8–12 h after pretreatment of AY9944 (100 nM) (c) or cariprazine (100 nM) (d) for 48 h in LPDS-supplemented medium. For Caki1, n = 2 biological replicates and for the other cell lines, n = 3 biological replicates. For a-d, data are representative of at least two independent experiments. Data are shown as the mean ± s.d. when n = 3 biological replicates (a, c, d).
Extended Data Fig. 6 7-DHC protects against phospholipid peroxidation.
a, Lipid peroxidation assessment of HEK293T treated with RSL3 (2.5 μM) for 2 h after pretreatment of 7-DHC and Fer-1. b, c, Lipid peroxidation assessment of HT1080 treated with RSL3 (1 μM) for 1.5 h after pretreatment of 7-DHC. d, Lipid peroxidation assessment of WT and CYP51A1, MSMO1, EBP, SC5D, DHCR7 KO HEK293T treated with RSL3 (2.5 μM) for 1 h. e. Heat map showing the representation of oxidized phospholipid in WT and DHCR7 KO HEK293T treated with or without RSL3 for 4 h. Each row represents z-score-normalized intensities of the detected lipid species. a, acyl; e, plasmanyl. f, g, Lipidomic profile of phosphatidylethanolamine (PE) (f) and phosphatidylcholine (PC) (g) of WT and DHCR7 KO HEK293T. h, Mitochondrial lipid peroxidation assessment of HT1080 treated with RSL3 (0.5 μM) and BQR (200 μM) for 2 h after pretreatment of 7-DHC (25 μM) and Fer-1 for 24 h. i, Mitochondrial lipid peroxidation assessment of WT and SC5D KO HEK293T treated with RSL3 (1 μM) and BQR (200 μM). j, Chemical structures of different sterols. Red arrows point to 5, 7-diene. k, Representative autoxidation inhibited by indicated concentrations of 7-DHC. l, Representative autoxidation inhibited by 50 μM indicated sterols. m, Cell viability of HEK293T treated with RSL3 (5 μM) for 8 h after pretreatment of indicated sterols for 24 h. n = 2 biological replicates. n, Cell viability of HEK293T treated with RSL3 for 8 h after pretreatment of 7-DHC (25 μM) or 7-DHD (25 μM) for 24 h. n = 2 biological replicates. o, Levels of 7-DHC in HEK293T treated with RSL3 (4.5 μM) for indicated time. p, Chemical structures of 7-DHC-derived oxysterol DHCEO. q, r, The relative mRNA level (q) and protein level(r) of GPX4, FSP1, SLC7A11 and ACSL4 in HEK293T treated with 7-DHC (25 μM) for 24 h. s, The relative mRNA levels of ACSL1, TF and SCD1 in HEK293T treated with 7-DHC (25 μM) for 24 h, then cultured in LPDS-supplemented medium with 7-DHC for 24 h. t, The protein levels of ACSL1, TF, SCD1, SREBP2 (p-SREBP2) and nuclear form of SREBP2 (n-SREBP2) in HEK293T. Cells were treated with 7-DHC (25 μM) or the ethanol control (Ctrl) for 24 h, then cultured in LPDS-supplemented medium with 7-DHC for 24 h. Data are representative of two (e, k, l) or three (a-d, h, i, m-t) independent experiments, one experiment (f, g). Data are mean ± s.d. of n = 3 biological replicates (a-e, h, i, o-s), n = 4 biological replicates (f, g). Statistical analysis was performed using one-way ANOVA (a, c, h, o, and q) or two-way ANOVA (i and s); ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 7 Regulation of 7-DHC level during ferroptosis.
a, DHCR7 location in cell plasma membrane (PM) was detected by immunoblotting. b, Cells expressing vector or 3HA-OMP25 were performed mitochondria immunoprecipitation, DHCR7 was detected in mitochondria by immunoblotting. c, The captured mitochondria were used for LC/MS detection. Proteins identified by the mass spectrometry. d, Detection of DHCR7 carbonylation in HEK293T cells expressed DHCR7-FLAG after induced with RSL3 (6 μM) following treatment of Lip-1 (10 μM). e-g, m-APA labelled carbonylation of DHCR7 in HEK293T cells treated with RSL3 (6 μM) (e), ML210 (10 μM) (f) and in RCC4 treated with Erastin (10 μM) (g) following treatment of Lip-1 (10 μM) or Fer-1 (1 μM). h, m-APA labelled carbonylation of DHCR7 in HEK293T cells treated with RSL3 (5 μM) for indicated time. Level of lipid peroxidation and cell death (TB positive, TB: Trypan Blue) from indicated time points are depicted below in grey scale. i, m-APA selectively labelled carbonylation on C380 of HEK293T cells treated with RSL3 (4 μM). j, Schema of DHCR7 and carbonylated DHCR7 enrichment and the enzyme activity detection by NADPH-dependent reduction. DHCR7 from DMSO (DHCR7-D1st) or RSL3 (DHCR7-R1st) treatment group were enriched by Flag IP. Carbonylated DHCR7 (DHCR7-R2nd) were enriched from DHCR7-R1st by biotin IP. k, Relative DHCR7 enzyme activity detected by the reduction of NADPH. l, m, A structural comparison between the crystal structure of the delta-14 sterol reductase from M. alcaliphilum (grey) and the human DHCR7 structure predicted by AlphaFold2 (cyan), the same residues are found at the NAPDH-binding pocket (red). n, Proposed model of regulation of 7-DHC level during ferroptosis. Image created with BioRender. For a-i, data are representative of at least two independent experiments. For k, data are mean ± s.d. of n = 4 biological replicates from two independent experiments. Statistical analysis was performed using one-way ANOVA (k), ***P < 0.001.
Extended Data Fig. 8 Targeting 7-DHC biosynthesis regulates cancer cell sensitivity to ferroptosis.
a, Levels of 7-DHC in various cancer cell lines treated with TASIN-30 (10 μM) for 8 h. b, Cell viability of various cancer cell lines treated with indicated concentrations of RSL3 for 8–10 h (SK-Hep1, HuH-7, PLC/PRF/5, U2OS and 786-O) or 18–24 h (HepG2, Hep3B, DLD1 and MDA-MB-231) following pretreatment of TASIN-30 (10 μM), Fer-1 (1 μM) or TASIN-30 (10 μM) plus Fer-1 (1 μM) for 4 h. c, Cell viability of WT, DHCR7 KO and EBP KO HEK293T cells treated with RSL3 for 8 h after pretreatment of TASIN-30 for 4 h. d, The fitness score of SC5D and DHCR7 in top 10 cell lines ranked by predicted dependence of 7-DHC. The predicted dependence of 7-DHC score is calculated by fitness score of DHCR7 minus fitness score of SC5D. e, Lipid peroxidation assessment of SU-DHL-8 cells treated with indicated concentrations of TASIN-30 for 16 h with pretreatment of Fer-1. f, Levels of 7-DHC in tumour tissue of SU-DHL-8 xenografts with indicated treatments. n = 7 in each group. g, Tumour weight of SU-DHL-8 xenografts with indicated treatments over time. n = 7 in each group. h, Body weight of SU-DHL-8 xenografts with indicated treatments over time. n = 7 in each group. Data plotted are mean ± s.e.m. i, Representative immunohistochemical staining of 4-HNE in tumour tissue of SU-DHL-8 xenografts with indicated treatments. Representative images of 7 different samples from a single experiment are shown. Scale bar, 50 μm. For (a-c and e), data are representative of three independent experiments (b) or two independent experiments (a, c, e), and data are mean ± s.d. of n = 3. For f-i, data are representative of two independent experiments (g, h) and one experiment (f, i), data plotted are mean ± s.e.m. and statistical analysis was performed using two-way ANOVA; **P < 0.01, ****P < 0.0001. Statistical analysis was performed using unpaired two-tailed t-test (a); ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 9 7-DHC promotes metastasis.
a, Levels of 7-DHC in B16F10 treated with 7-DHC (25 μM) for 24 h. b, Cell viability of B16F10 with treatment of RSL3 for 6–8 h following pretreatment of 7-DHC (25 μM) for 24 h. n = 2 biological replicates. c, d, Image (c) and quantification (d) of B16F10 metastatic tumours in lungs with cells pretreated 7-DHC (25 μM) for 24 h. n = 8 in each group. e, The protein level of Dhcr7 in sgNC and sgDhcr7 B16F10. f, Cell viability of sgNC and sgDhcr7 B16F10 treated with RSL3 for 6–8 h. n = 2 biological replicates. g, Levels of 7-DHC in sgNC and sgDhcr7 B16F10. h, i, Image (h) and quantification (i) of sgNC and sgDhcr7 B16F10 metastatic tumours. n = 8 in each group. j, k, Tumour volume (j) and tumour weight (k) of sgNC and sgDhcr7 B16F10 subcutaneous tumour. n = 7 in each group,. l, Cell viability of sgNC and sgDhcr7 B16F10 treated with RSL3 for 10–12 h following pretreatment of TASIN-30 (10 μM) for 24 h. m, Levels of 7-DHC in sgNC and sgDhcr7 B16F10 treated with TASIN-30 (10 μM) for 24 h. n, o, Image (n) and quantification (o) of metastatic tumours of sgNC and sgDhcr7 B16F10 pretreated TASIN-30 (10 μM) for 24 h. n = 8 in each group. p, q, Image (p) and quantification (q) of metastatic tumours of B16F10 in mice pre-injected with vehicle or AY9944 (25 mg/kg) via i.p. once daily for four times prior to i.v. of B16F10. n = 9 in each group. For a-q, data are representative of two independent experiments. Data are mean ± s.d. of n = 3 biological replicates (a, g, i, m) for cell experiments and mean ± s.e.m for animal experiments, statistical analysis was performed using unpaired two-tailed t-test (a, d, g, i, k and q) or two-way ANOVA (m and o); **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant.
Extended Data Fig. 10 Cariprazine protects IRI in vivo.
a, Levels of 7-DHC in serum of mice intraperitoneal injected of 7-DHC after indicated time. n = 3 in each group. b, Levels of 7-DHC in serum of mice kidney IRI model with pretreatment of AY9944. n = 7 in each group. c, Workflow of mice kidney IRI model with pretreatment of indicated dose of cariprazine via intraperitoneal injection once daily for four times before IRI. d, e, Levels of 7-DHC in serum (d) and kidneys (e) of mice kidney IRI model with pretreatment of indicated dose of cariprazine. f, Body weight of IRI model mice with indicated treatments over time. g, h, The concentration of BUN (g) and SCr (h) of mice IRI model. i, Workflow of IRI model with pretreatment of cariprazine at the dose of 5 mg/kg via intraperitoneal injection once daily for four times before IRI. j, Body weight of IRI model mice with indicated treatment over time. k, l, The concentration of BUN (k) and SCr (l) of mice kidney IRI model with indicated treatment over time. m, Levels of 7-DHC in kidneys of mice IRI model with pretreatment of cariprazine. n, o, Representative PAS staining (n) and quantification of renal damage (o) from mice IRI model. Scale bar, 100 μm. For d-h, n = 3 in each group, and for i-o, n = 7 in each group. For (a and d-o), data plotted are mean ± s.e.m. from one experiment. For b, data are representative of two independent experiments and data plotted are mean ± s.e.m. Statistical analysis was performed using one-way ANOVA (a, d-h) and two-way ANOVA (b, j-m, o), *P < 0.05, **P < 0.01 ***P < 0.001, ****P < 0.0001, ns, not significant. p, Working model of 7-DHC. 7-dehydrocholesterol (7-DHC) protects cells from lipid peroxidation on both plasma membrane and mitochondria via diverting the peroxidation pathway from phospholipids, thus mitigating ferroptosis. PLOOH, phospholipid hydroperoxide; PLOO·, phospholipid hydroperoxyl radical. Image created with BioRender.
Supplementary information
Supplementary Information
Supplementary Fig. 1: an example of the gating strategy for the lipid peroxidation and mitochondrial lipid peroxidation assays. Supplementary Fig. 2: raw images of immunoblotting.
Supplementary Tables 1–4
Supplementary Table 1: CRISPR screen data. Supplementary Table 2: sgRNA sequences. Supplementary Table 3: IC50 summary. Supplementary Table 4: P-value summary.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Ran, Q., Duan, Q. et al. 7-Dehydrocholesterol dictates ferroptosis sensitivity. Nature 626, 411–418 (2024). https://doi.org/10.1038/s41586-023-06983-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06983-9
This article is cited by
-
Natural inhibitor found for cell death by ferroptosis
Nature (2024)
-
B-ring sterols to the rescue
Nature Reviews Cancer (2024)
-
Cholesterol business: life or death by rust
Signal Transduction and Targeted Therapy (2024)
-
7-Dehydrocholesterol is an endogenous suppressor of ferroptosis
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.