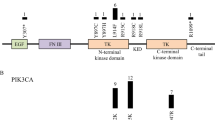
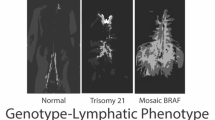
Abstract
Vascular anomalies are malformations or tumors of the blood or lymphatic vasculature and can be life-threatening. Although molecularly targeted therapies can be life-saving, identification of the molecular etiology is often impeded by lack of accessibility to affected tissue samples, mosaicism or insufficient sequencing depth. In a cohort of 356 participants with vascular anomalies, including 104 with primary complex lymphatic anomalies (pCLAs), DNA from CD31+ cells isolated from lymphatic fluid or cell-free DNA from lymphatic fluid or plasma underwent ultra-deep sequencing thereby uncovering pathogenic somatic variants down to a variant allele fraction of 0.15%. A molecular diagnosis, including previously undescribed genetic causes, was obtained in 41% of participants with pCLAs and 72% of participants with other vascular malformations, leading to a new medical therapy for 63% (43/69) of participants and resulting in improvement in 63% (35/55) of participants on therapy. Taken together, these data support the development of liquid biopsy-based diagnostic techniques to identify previously undescribed genotype–phenotype associations and guide medical therapy in individuals with vascular anomalies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
De-identified next-generation sequencing data for participants are available through controlled access at dbGaP with accession number phs003197.v1.p1. Data will be made available for secondary research only after investigators have obtained approval from the NIH to use the requested data for a particular project. Source data are provided with this paper.
References
Makinen, T., Boon, L. M., Vikkula, M. & Alitalo, K. Lymphatic malformations: genetics, mechanisms and therapeutic strategies. Circ. Res. 129, 136–154 (2021).
Queisser, A., Seront, E., Boon, L. M. & Vikkula, M. Genetic basis and therapies for vascular anomalies. Circ. Res. 129, 155–173 (2021).
Wassef, M. et al. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics 136, e203–e214 (2015).
Li, D. et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat. Med. 25, 1116–1122 (2019).
Adams, D. M. et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics 137, e20153257 (2016).
Ricci, K. W. et al. Efficacy of systemic sirolimus in the treatment of generalized lymphatic anomaly and Gorham-Stout disease. Pediatr. Blood Cancer 66, e27614 (2019).
Hammill, A. M. et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr. Blood Cancer 57, 1018–1024 (2011).
Triana, P. et al. Sirolimus in the treatment of vascular anomalies. Eur. J. Pediatr. Surg. 27, 86–90 (2017).
Foster, J. B. et al. Kaposiform lymphangiomatosis effectively treated with MEK inhibition. EMBO Mol. Med. 12, e12324 (2020).
Dori, Y. et al. Severe lymphatic disorder resolved with MEK inhibition in a patient with Noonan syndrome and SOS1 mutation. Pediatrics 146, e20200167 (2020).
Chowers, G., et al. Treatment of severe kaposiform lymphangiomatosis positive for NRAS mutation by MEK inhibition. Pediatr Res. https://doi.org/10.1038/s41390-022-01986-0 (2022).
Pfeiffer, F. et al. Systematic evaluation of error rates and causes in short samples in next-generation sequencing. Sci. Rep. 8, 10950 (2018).
Smith, T., Heger, A. & Sudbery, I. UMI-tools: modeling sequencing errors in unique molecular identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017).
Liu, M. et al. Genetics etiologies and genotype phenotype correlations in a cohort of individuals with central conducting lymphatic anomaly. Eur. J. Hum. Genet. 30, 1022–1028 (2022).
Mackie, A. S., Veldtman, G. R., Thorup, L., Hjortdal, V. E. & Dori, Y. Plastic bronchitis and protein-losing enteropathy in the Fontan patient: evolving understanding and emerging therapies. Can. J. Cardiol. 38, 988–1001 (2022).
Dori, Y. & Smith, C. L. Lymphatic disorders in patients with single ventricle heart disease. Front Pediatr. 10, 828107 (2022).
Ghosh, R. M. et al. Prevalence and cause of early Fontan complications: does the lymphatic circulation play a role? J. Am. Heart Assoc. 9, e015318 (2020).
Li, D. et al. Pathogenic variant in EPHB4 results in central conducting lymphatic anomaly. Hum. Mol. Genet. 27, 3233–3245 (2018).
Byrne, A. B. et al. Pathogenic variants in MDFIC cause recessive central conducting lymphatic anomaly with lymphedema. Sci. Transl. Med. 14, eabm4869 (2022).
Li, D. et al. Expanded phenotypic spectrum of JAG1-associated diseases: central conducting lymphatic anomaly with a pathogenic variant in JAG1. Clin. Genet. 99, 742–743 (2021).
Konczyk, D. J. et al. Arteriovenous malformation MAP2K1 mutation causes local cartilage overgrowth by a cell-non autonomous mechanism. Sci. Rep. 10, 4428 (2020).
Zenner, K. et al. Cell-free DNA as a diagnostic analyte for molecular diagnosis of vascular malformations. Genet. Med. 23, 123–130 (2021).
Sun, Y. et al. Cell-free DNA from plasma as a promising alternative for detection of gene mutations in patients with Maffucci syndrome. Hereditas 159, 4 (2022).
Wiggins, J. M., Ali, S. & Polsky, D. Cell-free DNA in dermatology research. J. Invest. Dermatol. 142, 1523–1528 e1521 (2022).
Palmieri, M. et al. Cell-free DNA next-generation sequencing liquid biopsy as a new revolutionary approach for arteriovenous malformation. JVS Vasc. Sci. 1, 176–180 (2020).
Biderman Waberski, M. et al. Urine cell-free DNA is a biomarker for nephroblastomatosis or Wilms tumor in PIK3CA-related overgrowth spectrum (PROS). Genet. Med. 20, 1077–1081 (2018).
Chen, W. L. et al. The utility of cerebrospinal fluid-derived cell-free DNA in molecular diagnostics for the PIK3CA-related megalencephaly-capillary malformation (MCAP) syndrome: a case report. Cold Spring Harb. Mol. Case Stud. 8, a006188 (2022).
Allen-Rhoades, W. et al. Cellular variant of kaposiform lymphangiomatosis: a report of three cases, expanding the morphologic and molecular genetic spectrum of this rare entity. Hum. Pathol. 122, 72–81 (2022).
Barclay, S. F. et al. A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genet. Med. 21, 1517–1524 (2019).
Schuart, C. et al. Cutis marmorata telangiectatica congenita being caused by postzygotic GNA11 mutations. Eur. J. Med. Genet. 65, 104472 (2022).
Rofes, P. et al. Mosaicism in PTEN-new case and comment on the literature. Eur. J. Hum. Genet. 30, 641–644 (2022).
Zhou, X. P. et al. Germline and germline mosaic PTEN mutations associated with a Proteus-like syndrome of hemihypertrophy, lower limb asymmetry, arteriovenous malformations and lipomatosis. Hum. Mol. Genet. 9, 765–768 (2000).
Caux, F. et al. Segmental overgrowth, lipomatosis, arteriovenous malformation and epidermal nevus (SOLAMEN) syndrome is related to mosaic PTEN nullizygosity. Eur. J. Hum. Genet. 15, 767–773 (2007).
Konczyk, D. J. et al. Arteriovenous malformation associated with a HRAS mutation. Hum. Genet. 138, 1419–1421 (2019).
Rodriguez-Laguna, L. et al. Somatic activating mutations in PIK3CA cause generalized lymphatic anomaly. J. Exp. Med. 216, 407–418 (2019).
Sheppard, S. E. et al. Lymphatic disorders caused by mosaic, activating KRAS variants respond to MEK inhibition. JCI Insight https://doi.org/10.1172/jci.insight.155888 (2023).
Homayun-Sepehr, N. et al. KRAS-driven model of Gorham-Stout disease effectively treated with trametinib. JCI Insight 6, e149831 (2021).
Nozawa, A. et al. A somatic activating KRAS variant identified in an affected lesion of a patient with Gorham-Stout disease. J. Hum. Genet. 65, 995–1001 (2020).
Chang, C. A. et al. Novel findings and expansion of phenotype in a mosaic RASopathy caused by somatic KRAS variants. Am. J. Med. Genet. A 185, 2829–2845 (2021).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012).
Dunn, T. et al. Pisces: an accurate and versatile variant caller for somatic and germline next-generation sequencing data. Bioinformatics 35, 1579–1581 (2019).
Wu, L. R., Chen, S. X., Wu, Y., Patel, A. A. & Zhang, D. Y. Multiplexed enrichment of rare DNA variants via sequence-selective and temperature-robust amplification. Nat. Biomed. Eng. 1, 714–723 (2017).
Villefranc, J. A., Amigo, J. & Lawson, N. D. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 (2007).
Jung, H. M. et al. Development of the larval lymphatic system in zebrafish. Development 144, 2070–2081 (2017).
Acknowledgements
We thank the patients and their families for participating in the research. We also thank the Comprehensive Vascular Anomaly Program for clinical care. The work was supported by a Children’s Hospital of Philadelphia Frontier Program Grant (D.M.A., H.H.), Children’s Hospital of Philadelphia K-Readiness Grant (S.E.S.), the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number 5R21TR003331 (D.L.), a research grant from the Lymphatic Malformation Institute (D.L.), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number ZIA-HD009003-01 (S.E.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank A. Hoofring of NIH Medical Arts for his assistance with the preparation of Fig. 2c.
Author information
Authors and Affiliations
Contributions
D.L., S.E.S. and H.H. conceived the study. D.L., S.E.S., A.J.B., F.W., M.E.M., C.S. designed the methodology. M.E.M., M.R.B., L.S.M., C.S., M.C.M. and S.M.P. performed functional investigations. D.L., S.E.S., M.E.M., L.S.M., L.F.S., A.S.S., G.K., E.P., A.J.B., A.D.B., L.B., M.Q., J.R.R., J.A.N., D.W.L., S.V., J.T., C.L.S., A.M.C., K.M.S., D.M.A., Y.D. and H.H. carried out formal analysis. D.L., S.E.S., M.E.M., L.S.M., L.F.S., A.S.S., A.J.B., E.P., A.D.B., M.Q., J.R.R., J.A.N., D.W.L., S.V., J.T., C.L.S., A.M.C., K.M.S., D.M.A., Y.D., H.H., L.T., F.W., J.D., R.P.S., S.N.C., C.H., C.K. and K.N. conducted investigation. D.L., S.E.S., M.E.M., L.S.M., L.F.S., A.S.S., E.P., A.J.B., M.Q., J.R.R., J.A.N., D.W.L., S.V., J.T., C.L.S., A.M.C., K.M.S., D.M.A., Y.D., H.H. and J.D. performed data curation. D.L., S.E.S., L.F.S., A.S.S., A.J.B., S.R., J.D., M.E.M. and C.S. provided visualization. B.N., T.S., N.O., A.T. and L.W. carried out project administration. D.L. and S.E.S. wrote the original draft. All authors performed reviewing and editing. D.L., S.E.S., M.C., D.M.A., Y.D. and H.H. provided supervision. D.L., S.E.S., D.M.A., Y.D. and H.H. acquired funding. D.M.A., Y.D. and H.H. contributed equally to this manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.H. and CHOP are equity holders in Nobias Therapeutics, which is developing MEK inhibitor therapy for complex lymphatic anomalies. D.M.A. is a consultant for Novartis Pharmaceuticals and Nobias. K.M.S. is a consultant for Novartis Pharmaceuticals, which makes Vijoice (alpelisib), a selective PI3Ka inhibitor. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Douglas Marchuk and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling editor: Anna Maria Ranzoni, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Functional characterization of BRAF-F486S, RAF1-T145P, and KRAS-A146T.
Transduction of BRAF-F486S (a) or RAF1-T145P (c) significantly increased the level of p-ERKs and Trametinib treatment led to a significant reduction of p-ERKs. Three-dimensional lymphatic spheroid sprouting assay showed elevated sprouting activity in HDLECs expressing BRAF-F486S (b) or RAF1-T145P (d) compared to its WT as measured by both cumulative sprout length and number of sprouts. Trametinib treatment led to a significant reduction of both cumulative sprout length and number of sprouts. For a-d, three independent experiments were performed. P values were calculated using 2-sided t-tests and included in each panel (degree of freedom was included in the Source Data for Extended Data Fig. 1), and corrected for multiple testing using the FDR (Benjamini and Hochberg) method. Bar graphs in panels a and c represent mean fold change in the ratio of pERK to GAPDH, normalized to the untreated wild type. Error bars represent standard deviations. In the box and whisker plots in panels b and d, the line in the middle of the box represents the medians, tops and bottoms of the boxes represent the 25th and 75th quartiles respectively, and the whiskers extend to 1.5 times the interquartile range beyond the 25th and 75th quartiles. All the data points that are summarized by the boxplots are superimposed as dots on the plots; minima and maxima can be determined by the highest and lowest dots. e, e’, Expression of KRASWT had no impact on lymphatic vessel morphology in trunk. f, f’, Expression of KRASA146T resulted in lymphatic tissue expansion (asterisks) and dilation of the thoracic duct (dotted line). Red: Expression of transgene in trunk of zebrafish at 5dpf, Green: mrc1a:GFF labeling lymphatic vessels. g, Quantitation of WT EK larvae that were assayed for pericardial edema at 5 dpf. Injected embryos were screened for transgenic mCherry expression in endothelial cells prior to quantitation so that only transgenic expressing embryos were counted. The p-values in the graph were calculated via Fisher’s Exact tests (two-sided) between samples as indicated, followed by Bonferroni correction. Indicated n are the total number of larvae assayed per condition.
Supplementary information
Supplementary Table 1
Molecular findings for the cohort and validation.
Supplementary Table 2
Molecular findings in blood or saliva samples in patients who typically have germline variants.
Supplementary Table 3
Molecular findings from deep exome sequencing.
Supplementary Table 4
Molecular findings from UMI panel ultra-deep sequencing.
Supplementary Table 5
Molecular findings from FFPE samples.
Supplementary Table 6
Molecular findings from body fluid samples.
Supplementary Table 7
Molecular findings from cfDNA.
Supplementary Table 8
PIK3CA variants were identified in 45 patients with blood and lymphatic malformations.
Supplementary Table 9
BDA and qPCR oligonucleotide sequences.
Source data
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, D., Sheppard, S.E., March, M.E. et al. Genomic profiling informs diagnoses and treatment in vascular anomalies. Nat Med 29, 1530–1539 (2023). https://doi.org/10.1038/s41591-023-02364-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02364-x
This article is cited by
-
Nanopore DNA sequencing technologies and their applications towards single-molecule proteomics
Nature Chemistry (2024)