Abstract
Two in every five patients with active tuberculosis (TB) remain undiagnosed or unreported. Therefore community-based, active case-finding strategies require urgent implementation. However, whether point-of-care (POC), portable battery-operated, molecular diagnostic tools deployed at a community level, compared with conventionally used POC smear microscopy, can shorten time-to-treatment initiation, thus potentially curtailing transmission, remains unclear. To clarify this issue, we performed an open-label, randomized controlled trial in periurban informal settlements of Cape Town, South Africa, where we TB symptom screened 5,274 individuals using a community-based scalable mobile clinic. Some 584 individuals with HIV infection or symptoms of TB underwent targeted diagnostic screening and were randomized (1:1) to same-day smear microscopy (n = 296) or on-site DNA-based molecular diagnosis (n = 288; GeneXpert). The primary aim was to compare time to TB treatment initiation between the arms. Secondary aims included feasibility and detection of probably infectious people. Of participants who underwent targeted screening, 9.9% (58 of 584) had culture-confirmed TB. Time-to-treatment initiation occurred significantly earlier in the Xpert versus the smear-microscopy arm (8 versus 41 d, P = 0.002). However, overall, Xpert detected only 52% of individuals with culture-positive TB. Notably, Xpert detected almost all of the probably infectious patients compared with smear microscopy (94.1% versus 23.5%, P = <0.001). Xpert was associated with a shorter median time to treatment of probably infectious patients (7 versus 24 d, P = 0.02) and a greater proportion of infectious patients were on treatment at 60 d compared with the probably noninfectious patients (76.5% versus 38.2%, P < 0.01). Overall, a greater proportion of POC Xpert-positive participants were on treatment at 60 d compared with all culture-positive participants (100% versus 46.5%, P < 0.01). These findings challenge the traditional paradigm of a passive case-finding, public health strategy and argues for the implementation of portable DNA-based diagnosis with linkage to care as a community-oriented, transmission-interruption strategy. The study was registered with the South African National Clinical Trials Registry (application ID 4367; DOH-27-0317-5367) and ClinicalTrials.gov (NCT03168945).
Similar content being viewed by others
Main
TB is the world’s second leading cause of death by an infectious disease after COVID-19, contributing to ~1.5 million deaths in 2020 (ref. 1). Despite advances in TB diagnostics, ~4 million patients (almost 40%) remain undiagnosed or unreported globally, most of whom reside in periurban informal settlements of large cities in Africa and Asia1,2. The COVID-19 epidemic has worsened this situation, with case detection plummeting by ~30–50%3,4. The ‘missing’ patients are important to detect and treat, and hence crucial for TB control, because they serve as a potential reservoir for transmission of drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis5. Indeed, modeling studies have indicated that a substantial reduction in transmission, and hence disease burden and mortality, will require community-based, active case finding (ACF; healthcare worker seeks, identifies and procures samples for TB testing in the community) rather than passive case finding (patient-driven self-presentation at a healthcare facility), which detects cases after most transmission has already occurred2,6,7,8.
There are several approaches to performing ACF in high-prevalence settings, including targeted screening of high-risk groups (for example, close contacts of TB index cases)9,10,11, community-based door-to-door screening6,11,12,13 and community-based screening using a mobile unit or clinic14,15. Door-to-door ACF using laboratory-based molecular tools such as the Cepheid GeneXpert system has recently been shown to have favorably impacted disease burden in the wider community6. However, the door-to-door ACF strategy is labor intensive and may not be affordable in many settings. Corbett et al., before the availability of automated molecular diagnostic tools, showed that both door-to-door and a mobile unit-based screening strategy favorably impacted disease burden at the community level, but the latter was more efficient14. Advancing these findings, we showed, in a randomized controlled trial, that a mobile clinic/unit-based approach using POC molecular tools was feasible and effective using a high-cost truck equipped with generator-powered GeneXpert16. Two subsequent cross-sectional studies showed that mobile clinic-based ACF approaches using molecular tools were feasible, with a number-needed-to-screen (NNS, that is, number of individuals needed to be screened to diagnose 1 case of TB from between 15 and 20 (refs. 15,17)). Notably, these studies were personnel intensive (staffed by doctors15,17, nurses15,17, laboratory technicians17 and radiographers15,17) and used relatively high-cost, nonscalable approaches including electricity-driven mobile laboratories with capacity to perform on-site smear microscopy, molecular testing and/or chest radiography17, limiting their applicability in TB-endemic settings. Controlled trials to evaluate the utility of molecular tools at a primary care level have, hitherto, not been undertaken. Indeed, the recent World Health Organization (WHO) guidance on systematic screening18 and a recent systematic review19 have underscored the need for well-designed studies to elucidate which ACF delivery methods and diagnostic strategies are most effective.
More recently, a portable battery-operated version of GeneXpert has become available (GeneXpert Edge) that is ideally suited as a POC diagnostic which could be incorporated into a scalable and affordable ACF model. We evaluated the feasibility of such a model (designated XACT) incorporating a two-person mobile clinic using a low-cost multipurpose vehicle, minivan (<US$12,000), equipped with GeneXpert Edge. An imperative of any such approach is to detect not only all the TB patients but also all the infectious patients who drive transmission. Therefore, in the present study, we prioritized evaluating the ACF model’s efficacy to detect infectious patients, which we identified using a combination of new cough aerosol sampling technology, chest X-ray characteristics and smear-microscopy status. Given this imperative we compared the molecular strategy with a same-day smear-microscopy strategy for several reasons. First, and most importantly, at the time of study design smear microscopy was the standard of care in primary health settings in TB-endemic countries, and remains so in many African countries. More recent WHO guidance (2021) recommends GeneXpert for active case finding in people who are not infected with human immunodeficiency virus (HIV) at the discretion of the healthcare provider, because the available evidence is of very low quality and based on a small dataset18 and the use of symptom screening with smear microscopy is endorsed in resource-poor settings20. Second, at the time the study was planned, we hypothesized that smear microscopy would detect infectious patients to the same extent as GeneXpert (given the much higher mycobacterial burden in presumed infectious patients, most of whom would probably be smear positive). Third, although GeneXpert has clearly been shown to be more sensitive than smear microscopy in numerous large clinic- and hospital-based studies21,22, there are limited data from paucibacillary populations (for example, with minimal disease burden as is commonly seen in the context of ACF). In such a subpopulation, GeneXpert testing might miss a substantial proportion of low-burden disease (mycobacterial load below the detection limit of the assay), thus significantly negating its intuitively higher sensitivity or beneficial effect23. Fourth, GeneXpert’s utility might be negated by false-positive readouts within those with previous TB (~20% of those presenting with symptoms in many endemic settings) and, finally, GeneXpert-positive people (because they are probably minimally symptomatic) are more likely to decline or fail to continue with treatment. Collectively, the rationale and equipoise underpinned by these reasons mandated why same-day smear microscopy was used as the comparator in our trial. Thus, in summary, the primary aim was to determine whether GeneXpert led to significantly shorter time-to-treatment initiation, and the key secondary aims were to determine whether GeneXpert identified a higher proportion of TB (especially infectious TB) and whether the scalable XACT model using advanced portable genomic technology was feasible in a TB-endemic setting. Our findings confirmed that POC GeneXpert, given its higher sensitivity and propensity to deliver a result more quickly (within 90 min), was associated with a more rapid time-to-treatment initiation, detected a higher proportion of patients (albeit only 50% of the total burden), detected almost all the probably infectious cases and was feasible in a resource-poor endemic setting.
Trial population
A total of 5,274 participants were screened for TB between 15 November 2016 and 15 February 2019. The demographic characteristics of the participants are shown in Table 1.
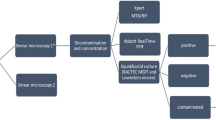
Figure 1 outlines the consort diagram, which includes 596 participants; 295 and 301 participants were randomized into the Xpert and smear arms, respectively. Of the participants who underwent randomization, seven were excluded from the Xpert group whereas five were excluded from the smear group because of lack of symptoms (one patient from each group) or being sputum scarce despite sputum induction (six patients in the Xpert arm and four in the smear arm). The remaining 288 participants in the Xpert group and 296 participants in the smear group were included in the analysis. Of participants, 11.1% (32 of 288) in the Xpert group and 8.8% (26 of 296) in the smear group had detectable growth on M. tuberculosis cultures from their sputum specimen. TB treatment was initiated in 56.3% (18 of 32) and 34.6% (9 of 26) in the Xpert and smear groups, respectively (including culture-only-based treatment initiation).
aPatients previously self-presented to a TB community clinic in the past 60 d or who had received treatment in the past 60 d. bUnable to consent (impaired/underage) or withdrew consent. cParticipants were recruited from their informal housing units due to an inability to walk to the mobile clinic on account of illness. dOnly the diagnostic test (and not culture) signaled treatment initiation. eEmpirical or culture-signaled treatment initiation. fBoth the diagnostic test and/or culture-signaled treatment initiation.
Patients randomized to the Xpert group rather than the smear group initiated treatment sooner
We first evaluated time-to-treatment initiation in each group (time from diagnostic testing to start of drug therapy within 60 d of diagnosis; see Fig. 1). The absolute time-to-treatment initiation (95% confidence interval (CI) (interquartile range (IQR))) was significantly shorter in the Xpert compared with the smear group (8 (4.3–29.5) versus 41 (24.0–71.5) d; P = 0.002) (Table 2). Comparatively, Xpert was associated with a more rapid time-to-treatment initiation within 60 d of testing (restricted mean time lost (RMTL)) ratio: 2.4 (95% CI 1.3–4.5, P = 0.008; hazard ratio (HR): 2.3, 95% CI 1.0–5.1, P = 0.04 (Fig. 2a)). This shorter time-to-treatment initiation was irrespective of whether start of therapy was based on the same-day test result alone or a combination of same-day and culture result (Fig. 2). Although the proportional hazards assumption was violated (that is, lack of consistency in the hazard over the duration of time) the HR very closely approximated the RMTL ratio, and both were significant (Fig. 2a). The RMTL ratio indicates that the mean treatment time gained over 60 d was ~2.4× greater in the Xpert compared with the smear arm. Proportionally, Xpert detected more TB patients than smear microscopy (50.0% (16 of 32) versus 11.5% (3 of 26); P = 0.03). A greater proportion of participants needed to be screened in the smear group (n = 98.6) versus the Xpert group (n = 18.0) to detect one patient with TB. Of participants, 235 of 288 (81.6%) in the Xpert arm and only 42 of 296 (14.2%) in the smear arm received their results on the same day (P < 0.01). Overall, only 27 of 58 (46.5%) of those with microbiologically proven TB initiated treatment (including culture-only-based treatment initiation) within 60 d (Xpert arm 18 of 32 (56.3) versus smear-microscopy arm 9 of 26 (34.6); P = 0.10).
Including same-day diagnostic test (Xpert and smear microscopy result) and/or culture-signaled treatment initiation and only same-day diagnostic test. Xpert and smear microscopy result signaled treatment initiation (but excluding culture-only-based treatment initiation). a,b, The Schoenfeld test results indicated that the proportional hazards assumption was not met for both (a) (P = 0.009) and (b) (P = 0.004). Thus, the RMTL ratio was calculated as an alternative (a) (2.4: 95% CI 1.3–4.5, P = 0.008) and (b) (3.4: 95% CI 1.5–7.6, P = 0.004) and was found to closely approximate the HR for both (a) (2.3: 95% CI 1.1–4.9, P = 0.03) and (b) (3.0: 95% CI 1.2–6.9, P = 0.02). The significance of the HRs was tested using the likelihood ratio test, whereas RMTL ratios were tested using the RMTL log(ratio) test. The censor dashes in (b) represent patients who were initiated on treatment based on culture results only.
Xpert detected more infectious patients than smear microscopy
Next, we investigated the ability of each diagnostic strategy to detect the probably infectious cases, which we defined as smear positivity and/or cough aerosol positivity (Extended Data Fig. 1) and/or the presence of cavitary disease on a chest X-ray. Overall, chest X-ray evaluation was performed in 51 of 58 (87.9%) culture-positive patients and 9 of 51 (17.6%) had cavitary disease. The proportions of probably infectious and probably noninfectious culture-positive patients are shown in Fig. 3 and Table 3. Of all the culture-positive patients (n = 58), only 51 participants had all three diagnostic tests portending infectiousness (that is, cough aerosol sampling system (CASS) and chest X-ray and smear status); 17 of 51 participants were deemed infectious (positive by ≥1 of the three criteria) and 34 were deemed probably noninfectious (negative by all three criteria; Fig. 3). Overall, Xpert detected more infectious TB patients than smear microscopy (94.1% (16 of 17) versus 23.5% (4 of 17); P = 0.004; Table 3). This pattern remained consistent when defining infectiousness using different combinations and permutations, including by CASS and cavitary status alone (see Table 3). Notably, the median time-to-treatment initiation for infectious patients was significantly earlier in the Xpert arm (7 versus 24 d; P = 0.02, that is, 17 d earlier in the Xpert arm; Table 2).
aProportion of patients on treatment at 60 d was significantly greater in the infectious group compared with the probably noninfectious group (13 of 17 (76.5%) versus 13 of 34 (38.2%); P = 0.007). bGiven the limitation of current tools, a minority of these patients (<10%) may still have transmission potential. cComparison of two proportions was undertaken by using the Chi-squared test.
Feasibility of implementing the XACT model for active case finding
The diagnostic accuracy of POC Xpert performed by minimally trained healthcare workers was comparable to Xpert performed by a qualified technician in a research laboratory (sensitivity 0.52 versus 0.61 and specificity of 0.98 for both; P = 0.46; see Supplementary Table 1). A total of 36 of 494 (7.3%) d were lost due to logistical challenges (breakdowns, inclement weather, and so on); mechanical problems with the vehicle was the most common reason for the lost screening days (see Supplementary Table 2). A vast majority (235 of 288, 81.6%) of the participants in the Xpert arm of the study received their results on the same day, thus enabling ‘immediate’ (that is, same visit) referral for TB treatment initiation during the same interaction, whereas only a small proportion (42 of 296, 14.2%) of participants in the smear arm received their results on the same day (Extended Data Fig. 2).
Discussion
To our knowledge this is the first controlled trial to show that an ACF strategy using a mini-mobile, clinic-based, scalable intervention package, incorporating a low-cost minivan and portable battery-operated Xpert (that is, the XACT model), was feasible and more importantly detected most infectious TB patients. Significantly, these were patients who did not self-report to healthcare facilities. The number of at-risk people (with any TB symptom or HIV infected) who needed to be screened, to detect one case of active TB, was 18 for Xpert versus 99 for smear microscopy. Furthermore, POC Xpert detected a higher proportion of culture-positive TB patients who initiated treatment within the first 60 d posttesting, and significantly reduced time-to-treatment initiation compared with same-day smear microscopy performed at a nearby microscopy center (within a 5-km radius).
There are recent reports confirming the feasibility of using the Xpert Edge system at POC for the diagnosis of TB24,25. However, we have now established the feasibility of using Xpert Edge as part of a standardized intervention package for community-based ACF in TB-endemic settings. Indeed, there was good concordance between Xpert performed at POC by minimally trained healthcare workers versus Xpert performed at a centralized laboratory, the model was well accepted within communities (~98% of the invited participants were recruited) and only 7.3% (36 of 494) of days were ‘lost’ due to nonscreening (problems with the vehicle, bad weather, and so on; see Supplementary Table 2). Importantly, the model is scalable (more easily reproducible because of better affordability) compared with more expensive and retro-fitted larger trucks that are manned by more personnel, use a generator and larger Xpert modules, and are less suited to densely populated shanty towns with narrow roads and lanes.
Xpert performed at POC identified significantly more patients with culture-positive TB, reduced time-to-treatment initiation (8 versus 41 d) and enabled the initiation of treatment within 60 d of testing in a significantly larger proportion of culture-positive patients compared with same-day smear microscopy. This is mainly attributable to Xpert’s higher detection rate for culture-positive TB and its ability to facilitate prompt referral for treatment initiation during the same interaction (reducing pretreatment loss to follow-up). Delays in obtaining a same-day result (higher proportion in the smear group received results the following day) contributed negligibly to this effect.
The overall Xpert-based case detection among participants with HIV infection and/or symptoms suggestive of TB (targeted screened group) was high at ~10%, and higher than that found in the Philippines (6% targeted screened prisoners) and Nepal (5%; n = 1239 and using a minivan approach)15,26. This is probably attributable to the higher rate of HIV endemicity (~20% of the population) and socioeconomic deprivation in our population. Nevertheless, Xpert sensitivity in detecting culture-positive TB was only ~51% and was due to the paucibacillary nature of disease in this community-based cohort, as shown in other studies27.
Significantly, although detecting only 50% of culture-positive patients, Xpert was highly effective in detecting most potentially infectious patients (~90% compared with 25% with smear microscopy), and the median time to treatment of these probably infectious patients was 17 d earlier than in the smear arm. This is a critical point because one of the core overarching purposes of ACF is to interrupt transmission, which should hence detect most infectious patients. Xpert is better than smear microscopy in this respect. As there is no clear-cut definition of what constitutes infectiousness, we used a flexible and nonredundant approach incorporating the presence of smear positivity and/or cough aerosol positivity and/or cavitary disease. Our conclusions remained unchanged despite the use of different definitions (Table 3). Although cavitary disease is associated with transmission and smear positivity28,29, ~15% of all TB transmission within the community occurs in smear-negative people30,31, and only a minority (approximately one-third) of smear-positive people have detectable culture-positive cough aerosol (culturable bacilli in cough microdroplets <10 µm in size) often with minimal symptoms32. CASS is the only measure of infectiousness that correlates with clinically meaningful endpoints such as tuberculin skin test conversion and development of active TB within 2 years33,34.
Importantly, only 46.5% of culture-positive participants (although 100% of Xpert-positive people) were on TB treatment at 60 d. This was due to a combination of factors including disease stigmatization, social and health system factors that may have modulated access to treatment (for example, transport costs, impact on work), migration and the presence of minimal symptoms that may have made individuals more likely not to initiate or continue with treatment. Indeed, we found that a higher proportion of the patients who were probably infectious (more symptoms and more severe disease) were on treatment compared with those who were probably noninfectious. The pragmatic trial design meant that patients were provided with a single TB education session and a referral letter (with the results) linking them to care. Given these considerations, future studies and programmatic roll-out of ACF packages should include appropriate intensive follow-up and incentives to ensure that patients are properly linked to care.
Our study had several limitations. First, we screened only symptomatic patients and asymptomatic HIV-infected participants. However, the broader objective of our study was to validate a low-cost and scalable screening model, and WHO-endorsed symptom screening has a high negative predictive value35. Second, our study had a limited sample size, which may have limited generalizability (single-center study in an HIV-endemic setting) and did not measure the impact of the model on disease burden and mortality. In contrast to the pre-molecular era36 Xpert-oriented ACF has been shown to impact disease burden6. Third, we used smear microscopy, and not Xpert performed at a centralized laboratory, as the comparator arm. However, we have justified the rationale for this in detail, including that we first needed to establish with certainty the superiority of Xpert in a community setting where low-burden disease predominates, and a different trial (NCT04303104) is currently evaluating optimal placement of the Xpert platform (POC versus centralized), whereas another trial (NCT04303104) is evaluating the role of the model in subclinical TB.
In conclusion, community-based ACF using a scalable mobile health intervention package incorporating portable molecular diagnostics is feasible and detects most community-based, probably infectious TB patients. These data define a new standard of care for community-based ACF and inform ACF strategies in TB-endemic settings.
Methods
Approvals, trial design and participants
The present study was approved by the University of Cape Town’s Human research Ethics Committee (ref. 068/2016), University of Stellenbosch Research Ethics Committee: Biosafety and Environment Ethics (SU-BEE17-0002) and Baylor Scott & Wine Research Institute (016-176). This parallel-group, single-center, open-labeled, randomized control trial was performed in the periurban resource-poor Mitchel’s Plain and Klipfontein subdistricts of Cape Town, South Africa. Participant enrollment occurred from 15 November 2016 to 15 February 2019. The trial sites were selected because they each have a high density of informal settlements (~9,000 people per km2). A research nurse and a community healthcare worker screened potential participants at the mobile clinic (vehicle equipped with a portable awning, providing shelter, fold-up tables, HIV lateral flow testing capability and a small portable fold-up cubicle for privacy during sputum acquisition, which securely housed the portable GeneXpert Edge system). The initial rapid screen at the mobile clinic took ~5 min and comprised five questions related to the presence of TB symptoms and HIV status. Participants who provided written informed consent (aged >18 years) were randomized if they had at least one TB symptom for ≥2 weeks or were HIV infected, irrespective of symptoms (positive HIV test on-site or known by themselves to be HIV infected). Participants who had attended a TB clinic for their symptoms or those who were on TB treatment were excluded. Screening was clustered around community congregational settings, for example, outside community centers, shopping malls and churches. The study was registered with the South African Clinical Trials Registry (application ID 4367; DOH-27-0317-5367) and ClinicalTrials.gov (NCT03168945). The registration with SANCTR occurred before the recruitment of the first patient.
Randomization
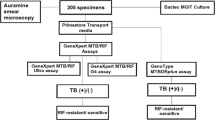
Participants who were eligible were randomized (1:1) to undergo either sputum smear microscopy (smear arm) at a nearby microscopy center (within ~5-km radius) or POC Xpert (Xpert arm) using a block size of 4. The clinical staff had access to TB culture results for all participants, but had access only to POC Xpert results for participants in the Xpert arm and to smear microscopy results for participants in the smear arm. The laboratory staff were blinded to the POC Xpert results and clinical details of the participants. Laboratory-based Xpert testing for all participants (in addition to the POC Xpert testing) was performed using bio-banked samples collected at the time of enrollment37.
Procedures
All specimen collection was performed at the POC. Xpert testing was performed by the trained research nurse/community health worker at the POC using GeneXpert Edge and Xpert Ultra cartridges. Sputum for smear microscopy (auramine staining) was collected and dispatched to a nearby microscopy center. The POC Xpert results were interpreted by the personnel performing the test whereas smear-microscopy results were emailed by the microscopy center to a designated clinical staff member as soon as they were available. A second sputum was collected for TB culture. A further sputum sample was bio-banked in both arms of the study (all sputum samples were collected over a period of 60 min). If participants could not produce sputum, sputum production was induced using a standard protocol41, which was also available as part of the mobile unit. If patients were diagnosed with TB, they were referred to the nearest clinic for treatment (within a 5-km radius) using a standardized referral template. Participants who tested positive in the Xpert arm of the study were referred to the TB clinic during the same visit whereas participants in the smear arm of the study were contacted by telephone to return to the mobile clinic, and subsequently referred to the TB clinic to initiate treatment (the referral letter contained the Xpert and smear-microscopy results, and participants were informed and advised about the nature of the disease and importance of initiating treatment). Sputum culture results were evaluated only 60 d after randomization for all participants (see Fig. 1). Participants who tested positive for TB on sputum culture were traced and given a referral letter to initiate TB treatment at the nearest TB clinic.
Feasibility of implementing the XACT ACF model
Xpert testing was performed on paired sputum samples (one test performed in the mobile van at the POC and the other in a research laboratory at the end of the study) to assess the competency of the healthcare workers who had no previous laboratory experience or training. The research nurse/community healthcare workers were trained on Xpert testing using a hybrid model (didactic lecture of ~1 h and a 2-h hands-on session). The trained staff were then paired with an experienced ‘Xpert tester’ in the field for a duration of 4–6 weeks to obtain hands-on experience. User appraisal questionnaires were also administered to assess staff competency in performing Xpert in the community at baseline (day of training), 4 weeks and 8 weeks. The number of ‘days lost’ due to technical factors (for example, equipment, staffing and vehicle) or environmental factors (for example, weather and sociopolitical factors) were also recorded. A ‘day lost’ was defined by the inability of the ACF team to perform any screening activities.
Determination of infectious patients
Infectious patients were defined as those who were expectorating a culture-positive aerosol (<10 μm) using CASS and/or who had acid-fast bacilli detectable on sputum smear microscopy and/or those with cavitary disease evident on chest X-ray. Smear-microscopy samples were processed in real time for participants in both arms of the study. However, the smear results for participants in the Xpert arm were withheld by the laboratory staff (that is, clinical staff and recruitment staff were blinded) up until the last participant had achieved the primary endpoint.
CASS assessment
All patients with positive results on sputum Xpert, sputum smear microscopy and/or sputum culture (when culture only resulted in treatment initiation) were evaluated with CASS at a separate facility before or within 48 h of starting treatment. This validated technique quantifies and size classifies cough aerosol particles that contain culturable M. tuberculosis42,43. Patients enter the cubicle and cough into the mouthpiece (Extended Data Fig. 1B). The cough particles flow, with the aid of a vacuum pump, to a chamber holding an Andersen six-stage cascade impactor (Extended Data Fig. 1A). Air is filtered and the CASS chamber is sterilized between each use. Extended Data Fig. 1C shows the cough aerosol capture plates after incubation. Aerosols from each stage of the impactor are deposited on to the plate holding the selective culture medium, mirroring the position of the filter apertures; each colony represents a footprint of probably one M. tuberculosis-containing infectious particle. Colony-forming units on each plate were enumerated.
Chest X-ray assessment
All patients with a positive result on sputum Xpert, sputum smear microscopy and/or sputum culture underwent a single standard posteroanterior projection chest X-ray assessment before or within 72 h of treatment.
Aims and outcome measures
The primary aim was to compare time to TB treatment initiation between the arms (arm-specific time to treatment initiation within 60 d of diagnostic testing was the primary outcome measure). This time threshold was chosen to allow for the results of sputum TB culture to become available. Secondary aims included: (1) feasibility of the XACT ACF model (evaluated by comparing performance of Xpert at the POC against that in the laboratory: user appraisal questionnaires and determination of days lost); (2) detection efficacy of potentially infectious patients; (3) time to treatment for probably infectious patients; (4) the proportion of culture-positive TB patients initiating TB treatment in each study arm; and (5) the NNS to detect a single patient with TB.
Statistical analysis
We reasoned that a total of 34 culture-positive participants (17 in each arm) would be required to demonstrate a 3.5-fold reduction in the time-to-treatment initiation assuming 95% confidence and 80% power, that is, assuming a risk ratio of 3.5 (that is, patients were likely to start treatment 3.5× sooner in the Xpert versus smear arm based on the findings of the XACT-1 study). We calculated that 500 at-risk participants would require targeted screening (that is, participants who were HIV infected or had at least one symptom of TB) after rapidly screening ~5,200 participants to detect at least 40 culture-positive TB patients (20 in each arm), taking into account a 10% loss to follow-up, a TB prevalence of 8% and a rapid screen:targeted screen ratio of 1:10. The probability of an event in the Xpert group relative to the smear group per unit time was expressed as an HR or RMTL ratio when the hazard assumption was not met38,39,40. Full details about sample-size derivations and statistical methods are provided in the online supplement. The nonparametric Wilcoxon’s rank-sum test was used to compare time to treatment initiation at each time-point. A time-to-event outcome analysis was performed with time-to-event curves compared using the log(rank) (Mantel–Cox) test. The proportionality of hazards was tested by inspection of Schonfeld residuals for each variable included. The restricted mean survival time (RMST) and the RMTL ratios were also calculated, although Cox’s proportional hazards assumptions were not explicitly met. We analyzed univariate and multivariate associations between time-to-treatment initiation using clinically important variables selected a priori for the model.
Kaplan–Meier curves covering the first 60 d after enrollment/randomization and transformed to display cumulative events were constructed to assess the time-to-treatment initiation in the smear and Xpert arms. Differences between the two groups were quantified by fitting Cox’s proportional hazards regression model and calculating the HR. The proportional hazards assumption for each covariate was tested by calculating the Schonfeld residuals. The RMST ratio and subsequently the RMTL ratio were also calculated as an alternative to Cox’s proportional hazards. The RMST and RMTL have no model assumptions and are therefore not subject to the restrictions imposed by the proportional hazards assumptions. The RMST is the average event-free time to a specified endpoint (60 d in this analysis) and is calculated by subtracting the RMST from the specified endpoint. The RMTL can approximate the HR and provides a more intuitive measure of treatment effects/benefits. Statistical analysis was performed using OpenEpi, SPSS 27.0.1 and R v.4.0.5.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Individual participant data will be made available to researchers who provide a protocol that is approved by their respective human research ethics committee. All protocols will be reviewed and approved by the XACT consortium trial steering committee up to 5 years after publication. A data sharing agreement will need to be concluded between the representatives of the requesting institution and the University of Cape Town Lung Institute. Data sharing requests should be directed to keertan.dheda@uct.ac.za.
References
World Health Organization. Global Tuberculosis Report (WHO, 2020).
World Health Organization. Systematic Screening for Active Tuberculosis: Principles and recommendations (WHO, 2013).
Madhi, S. A. et al. COVID-19 lockdowns in low- and middle-income countries: success against COVID-19 at the price of greater costs. S. Afr. Med. J. 110, 724–726 (2020).
Dheda, K. et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 10, 603–622 (2022).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. S2213-2600, 30079-6 (2017).
Marks, G. B. et al. Community-wide screening for tuberculosis in a high-prevalence setting. N. Engl. J. Med. 381, 1347–1357 (2019).
Azman, A. S., Golub, J. E. & Dowdy, D. W. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med. 12, 216 (2014).
Sun, A. Y. et al. Modeling the impact of alternative strategies for rapid molecular diagnosis of tuberculosis in Southeast Asia. Am. J. Epidemiol. 178, 1740–1749 (2013).
Balcha, T. T., Skogmar, S., Sturegård, E., Björkman, P. & Winqvist, N. Outcome of tuberculosis treatment in HIV-positive adults diagnosed through active versus passive case-finding. Glob. Health Action 8, 27048 (2015).
Kempker, R. R. et al. High yield of active tuberculosis case finding among HIV-infected patients using Xpert MTB/RIF testing. Open Forum Infect. Dis. 6, ofz233 (2019).
Rivera, V. R. et al. Diagnostic yield of active case finding for tuberculosis and HIV at the household level in slums in Haiti. Int. J. Tuberc. Lung Dis. 21, 1140–1146 (2017).
Lorent, N. et al. Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLoS ONE 9, e92754 (2014).
Chen, C. et al. Community-based active case finding for tuberculosis in rural western China: a cross-sectional study. Int. J. Tuberc. Lung Dis. 21, 1134–1139 (2017).
Corbett, E. L. et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 376, 1244–1253 (2010).
Morishita, F. et al. Bringing state-of-the-art diagnostics to vulnerable populations: the use of a mobile screening unit in active case finding for tuberculosis in Palawan, the Philippines. PLoS ONE 12, e0171310 (2017).
Calligaro, G. L. et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect. Dis. 17, 441–450 (2017).
Choun, K. et al. Performance of algorithms for tuberculosis active case finding in underserved high-prevalence settings in Cambodia: a cross-sectional study. Glob. Health Action 12, 1646024 (2019).
World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 2: screening—systematic screening for tuberculosis disease (WHO, 2021).
Burke, R. M. et al. Community-based active case-finding interventions for tuberculosis: a systematic review. Lancet Public Health 6, e283–e299 (2021).
World Health Organization, Regional Office for South-East Asia. Optimizing active case-finding for tuberculosis: implementation lessons from South-East Asia (WHO, 2021).
World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 3: diagnosis—rapid diagnostics for tuberculosis detection, 2021 update (WHO, 2021).
Davis, J. L., Cattamanchi, A., Cuevas, L. E., Hopewell, P. C. & Steingart, K. R. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 13, 147–154 (2013).
Meyer, A. J. et al. Sputum quality and diagnostic performance of GeneXpert MTB/RIF among smear-negative adults with presumed tuberculosis in Uganda. PLoS ONE 12, e0180572 (2017).
Nalugwa, T. et al. Readiness to implement on-site molecular testing for tuberculosis in community health centers in Uganda. Implement. Sci. Commun. 3, 9 (2022).
Cordeiro-Santos, M. et al. Feasibility of GeneXpert((R)) Edge for tuberculosis diagnosis in difficult-to-reach populations: preliminary results of a proof-of-concept study. Am. J. Trop. Med. Hyg. 103, 1065–1066 (2020).
Khanal, S. et al. Yield of intensified tuberculosis case-finding activities using Xpert® MTB/RIF among risk groups in Nepal. Public Health Action 6, 136–141 (2016).
Lawn, S. D. et al. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin. Infect. Dis. 54, 1071–1079 (2012).
Grzybowski, S., Barnett, G. D. & Styblo, K. Contacts of cases of active pulmonary tuberculosis. Bull. Int. Union Tuberc. 50, 90–106 (1975).
van Geuns, H. A., Meijer, J. & Styblo, K. Results of contact examination in Rotterdam, 1967–1969. Bull. Int. Union Tuberc. 50, 107–121 (1975).
Behr, M. A. et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353, 444–449 (1999).
Tostmann, A. et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin. Infect. Dis. 47, 1135–1142 (2008).
Theron, G. et al. Bacterial and host determinants of cough aerosol culture positivity in patients with drug-resistant versus drug-susceptible tuberculosis. Nat. Med. 26, 1435–1443 (2020).
Fennelly, K. P. et al. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Respir. Crit. Care Med. 169, 604–609 (2004).
Jones-Lopez, E. C. et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am. J. Respir. Crit. Care Med. 187, 1007–1015 (2013).
Menberu, M. A. Performance of the WHO 2011 TB symptom screening algorithm for pulmonary TB diagnosis among HIV-infected patients in Gondar University Referral Hospital, Ethiopia. Int. J. Microbiol. 2016, 9058109 (2016).
Kranzer, K. et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int. J. Tuberc. Lung. Dis. 17, 432–446 (2013).
Esmail, A., Tomasicchio, M., Meldau, R., Makambwa, E. & Dheda, K. Comparison of Xpert MTB/RIF (G4) and Xpert Ultra, including trace readouts, for the diagnosis of pulmonary tuberculosis in a TB and HIV endemic setting. Int. J. Infect. Dis. 95, 246–252 (2020).
Liang, F., Zhang, S., Wang, Q. & Li, W. Treatment effects measured by restricted mean survival time in trials of immune checkpoint inhibitors for cancer. Ann. Oncol. 29, 1320–1324 (2018).
Uno, H. et al. Adding a new analytical procedure with clinical interpretation in the tool box of survival analysis. Ann. Oncol. 29, 1092–1094 (2018).
Perego, C. et al. Utility of restricted mean survival time analysis for heart failure clinical trial evaluation and interpretation. JACC Heart Fail. 8, 973–983 (2020).
Peter, J. G., Theron, G., Singh, N., Singh, A. & Dheda, K. Sputum induction to aid diagnosis of smear-negative or sputum-scarce tuberculosis in adults in HIV-endemic settings. Eur. Respir. J. 43, 185–194 (2014).
Acuna-Villaorduna, C. et al. Cough-aerosol cultures of Mycobacterium tuberculosis in the prediction of outcomes after exposure. A household contact study in Brazil. PLoS ONE 13, e0206384 (2018).
Jones-Lopez, E. C. et al. Cough aerosols of Mycobacterium tuberculosis in the prediction of incident tuberculosis disease in household contacts. Clin. Infect. Dis. 63, 10–20 (2016).
Acknowledgements
This work was funded by the National Institutes of Health (grant no. CRDF-OISE-16-62105) and the South African Medical Research Council (grant no. RFA-EMU-02-2017). K.D.’s lab also acknowledges funding from the European and Developing Countries Clinical Trials Partnership (grant nos. TMA-2015SF-1043 and TMA-1051-TESAII), UK Medical Research Council (grant no. MR/S03563X/1) and the Wellcome Trust (grant no. MR/S027777/1). A.E. acknowledges funding from EDCTP (grant no. TMA-2015CDF-1052). The funders have not influenced the results of this trial in any way. We are indebted to the participants who took part in the this study. We thank the Health Directorate of the City of Cape Town for providing access to appropriate health care facilities. We further acknowledge the assistance of clinical staff at each of the TB clinics, the University of Cape Town Research Ethics Committee and community leaders and the University of Cape Town Lung Institute TB community advisory board for enabling this study. Lessons learned from patients during the conduct of the XACT-1 study were implemented in the design of the present study to facilitate acceptance of the XACT-2 active case finding model.
Author information
Authors and Affiliations
Contributions
K.D., A.E., P.R. and G.C. conceived the trial. K.D. was the principal investigator. K.D., A.E., P.R., S.O., M.T., A.P., R.M., E.M., R. W., M. K. and L.M. designed and performed the experiments. K.D., A.E., S.M., P.R., S.O., M.T. and A.P. analyzed the data and wrote the paper. All authors critically reviewed and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Gavin Churchyard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Farrell, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cough Aerosol Sampling System (CASS) used to measure culturable M. tuberculosis in cough aerosol droplets as a surrogate for infectiousness.
The CASS system consisting of a six-stage Anderson cascade impactor (ACI) showing the median expected droplet diameter (µm) for each stage (Panel A).Patient sitting in a negative pressure cubical while coughing into a mouth piece that is attached to the ACI (Panel B).Culture plate that enables CFUs from individual aerosol droplets to be isolated (Panel C) The ACI was horizontally contained in a 10 litre autoclavable chamber (panel D).
Extended Data Fig. 2 Overview of the recruitment activities and procedures for the XACT-II study.
* A vast majority (235/288; 81.6%) of the participants in the Xpert arm of the study received their results on the same-day,thus, enabling ‘immediate’ (that is, same-visit) referral for TB treatment initiation during the same interaction while only a small proportion (42/296; 14.2%) of participants in the smear arm received their results in the same-day.
Supplementary information
Supplementary Information
Supplementary Tables 1–6, one figure, background information and summary table describing the contents of the XACT ACF model.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esmail, A., Randall, P., Oelofse, S. et al. Comparison of two diagnostic intervention packages for community-based active case finding for tuberculosis: an open-label randomized controlled trial. Nat Med 29, 1009–1016 (2023). https://doi.org/10.1038/s41591-023-02247-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02247-1
This article is cited by
-
Multidrug-resistant tuberculosis
Nature Reviews Disease Primers (2024)