Abstract
Quantitative flow ratio (QFR) is a computation of fractional flow reserve (FFR) based on invasive coronary angiographic images. Calculating QFR is less invasive than measuring FFR and may be associated with lower costs. Current evidence supports the call for an adequately powered randomised comparison of QFR and FFR for the evaluation of intermediate coronary stenosis. The aim of the FAVOR III Europe Japan trial is to investigate if a QFR-based diagnostic strategy yields a non-inferior 12-month clinical outcome compared with a standard FFR-guided strategy in the evaluation of patients with intermediary coronary stenosis. FAVOR III Europe Japan is an investigator-initiated, randomised, clinical outcome, non-inferiority trial scheduled to randomise 2,000 patients with either 1) stable angina pectoris and intermediate coronary stenosis, or 2) indications for functional assessment of at least 1 non-culprit lesion after acute myocardial infarction. Up to 40 international centres will randomise patients to either a QFR-based or a standard FFR-based diagnostic strategy. The primary endpoint of major adverse cardiovascular events is a composite of all-cause mortality, any myocardial infarction, and any unplanned coronary revascularisation at 12 months. QFR could emerge as an adenosine- and wire-free alternative to FFR, making the functional evaluation of intermediary coronary stenosis less invasive and more cost-effective.
Introduction
Fractional flow reserve (FFR) and the instantaneous wave-free ratio (iFR) are the recommended tests for routine physiological assessment of intermediate coronary stenosis1. These pressure wire-based diagnostic methods were shown to provide favourable clinical outcomes in several randomised trials2345. The adoption of FFR has improved following the FAME studies56, but worldwide implementation remains heterogeneous and low. The reasons for this may include physicians’ confidence in visual assessment, the incremental and immediate costs, the longer procedure time, the risk related to the advancement of coronary guidewires, and the need for administration of adenosine7. To expand the use of physiological guidance for coronary interventions and to overcome the limitations of wire-based methods, invasive coronary angiography (ICA)-based computation methods were developed for less invasive physiological assessments891011.
Quantitative flow ratio (QFR) is a method for fast computation of FFR-equivalent measurements based on three-dimensional (3D) reconstructions of coronary arteries and an estimation of contrast flow velocity (eCFV) during ICA. The FAVOR II Europe Japan and FAVOR II China studies1213 validated the in-procedure feasibility and diagnostic performance of QFR computation against FFR and showed superiority compared with quantitative angiographic assessment (2D-QCA). The aim of the FAVOR III Europe Japan Study is to determine if a QFR-guided revascularisation strategy provides non-inferior 1-year clinical outcomes as compared to an FFR-guided revascularisation strategy.
Methods
The study is being performed in up to 40 international sites. Enrolment was initiated in November 2018, followed by multicentre enrolment from September 2019. Recruitment is expected to conclude by April 2023, and primary endpoint results will be reported in 2024. A study flowchart is shown in Figure 1. The study is listed at ClinicalTrials.gov: NCT03729739. At the point when the manuscript was submitted, 382 patients were enrolled.
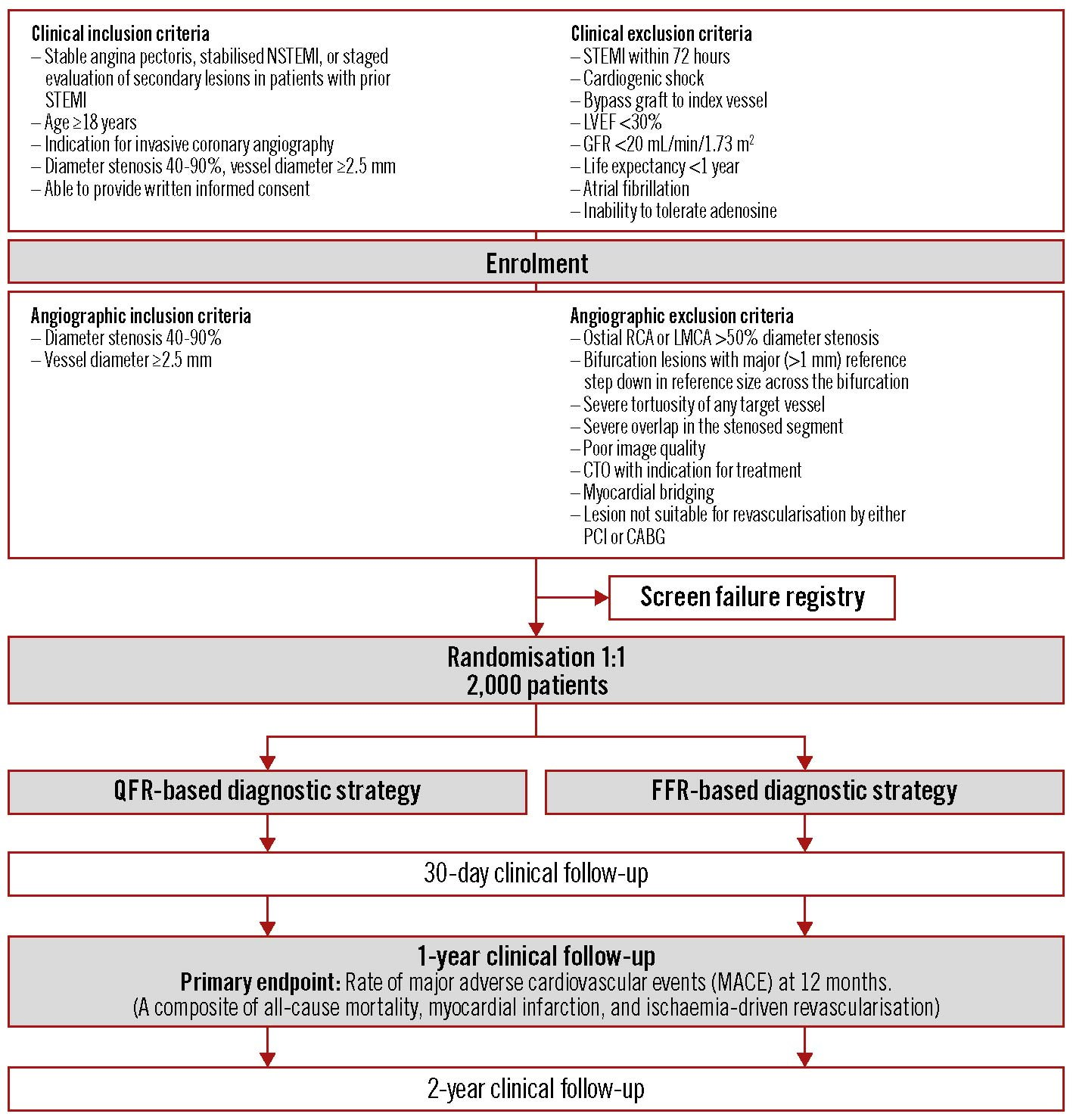
Figure 1. Study flowchart. CABG: coronary artery bypass graft; CTO: chronic total occlusion; FFR: fractional flow reserve; GFR: glomerular filtration rate; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; RCA: right coronary artery; STEMI: ST-elevation myocardial infarction.
Study hypothesis
A QFR-based diagnostic strategy yields a non-inferior clinical outcome as compared to a strategy using pressure wire-based FFR for the assessment of physiological significance and decision-making in patients with intermediate coronary artery stenosis.
Primary endpoints
The primary endpoint of major adverse cardiovascular events (MACE) is a composite of 1) all-cause mortality, 2) any myocardial infarction, and 3) any unplanned coronary revascularisation at 12 months.
Secondary endpoints
Secondary endpoints are listed in Supplementary Appendix 1. Procedural endpoints include performance endpoints on feasibility and procedure time. Lesion-specific documentation for ischaemia is required before repeat revascularisation in patients with angina pectoris. Clinical endpoints are reported for the 30-day, 12-month and 2-year follow-ups. An independent endpoint committee is adjudicating all possible events. Endpoint definitions are provided in Supplementary Appendix 1.
Patient population
Patients aged ≥18 with stable angina pectoris or with secondary evaluation of intermediate coronary artery stenosis after acute myocardial infarction and who are able to provide informed consent are eligible for enrolment. Clinical exclusion criteria include ST-segment elevation myocardial infarction (STEMI) within 72 hrs, severe kidney disease, allergy to contrast media or adenosine, atrial fibrillation at the time of ICA, coronary artery bypass graft (CABG) to any target vessel, or left ventricular ejection fraction (LVEF) <30%.
A diagnostic coronary angiography is performed after enrolment and angiographic in- and exclusion criteria have been checked. Patients are eligible for randomisation if they have at least 1 intermediate lesion (diameter stenosis of 40-90% by visual estimate in at least 2 angiographic projections) in a vessel with a reference diameter ≥2.5 mm. Key inclusion and exclusion criteria are provided in Figure 1. See Supplementary Appendix 1 for a full list of the criteria.
The population is described with baseline characteristics, anatomical parameters derived from QCA and the QFR or FFR distribution.
Randomisation
After evaluation of the initial angiographic runs, the patient is randomised in a 1:1 ratio to either QFR or FFR, if all angiographic inclusion criteria and no angiographic exclusion criteria are met (Supplementary Appendix 1).
Diagnostic strategy
All intermediate lesions in vessel segments with a reference size of at least 2.5 mm are assessed with the allocated method. Non-culprit lesions can be assessed in clinically stable non-ST-segment elevation myocardial infarction (NSTEMI) patients or in a staged evaluation (>72 hours) of patients initially admitted with STEMI.
Randomised patients are subsequently revascularised if indicated by the allocated functional test. Revascularisation is performed by either percutaneous coronary intervention (PCI) or CABG as determined by the clinician or the Heart Team, with the provision that patients with lesions deemed to be significant by the allocated diagnostic strategy undergo immediate or staged (within 60 days) complete coronary revascularisation (Figure 2). The diagnosis by the allocated diagnostic method should be adhered to independent of treatment strategy and whether the treatment is staged.
In cases with failed QFR or FFR evaluations, the patient may be diagnosed with the diagnostic method of the other arm. In the FFR group, any European conformity (CE)-marked resting index may be used as a bailout strategy, if the patient unexpectedly does not tolerate adenosine. Any crossover or use of bailout strategy is reported in measures of feasibility as unsuccessful QFR or FFR measurements.
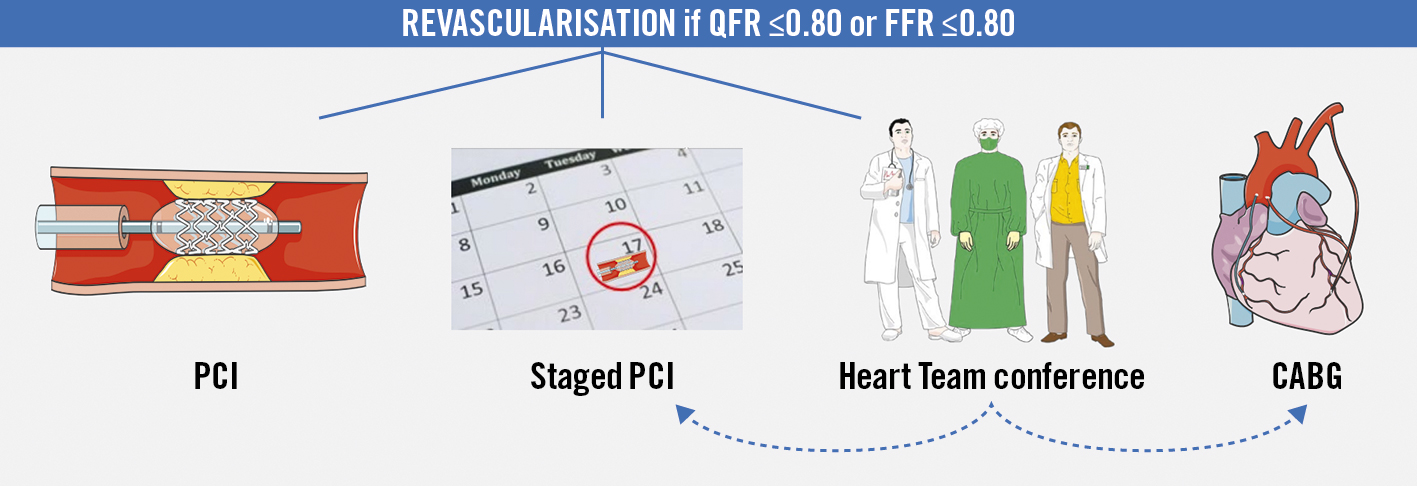
Figure 2. Coronary revascularisation. All stenoses assessed with either quantitative flow ratio (QFR) or fractional flow reserve (FFR) ≤0.80 should be revascularised. Options for revascularisation: 1) percutaneous coronary intervention (PCI), 2) staged PCI, 3) Heart Team conference leading to either staged PCI or coronary artery bypass grafting (CABG).
Ethics
The study has been notified to the local and regional ethics committees or institutional review boards as appropriate (Supplementary Appendix 1).
Study procedure
Invasive coronary angiography (ICA)
The ICA procedure follows current guidelines and local best practice14. The size and type of the catheters are left to the discretion of the treating physician, but the administration of nitroglycerine is mandatory. Two study projections at least 25 degrees apart are acquired for each lesion of interest, at a minimum of 12.5 frames/sec, before randomisation. A table of recommended projection angles is provided for all study sites. The study acquisitions should meet the demands for QFR analysis in both arms, i.e., aiming for minimal foreshortening and overlap, avoiding panning and zooming, and ensuring good contrast filling by a long and brisk injection. If the lesion morphology and angiographic quality meet the predefined criteria, the patient is randomised to either the QFR- or the FFR-guided strategy.
QFR allocation
A description of the QFR computation algorithm is found in Supplementary Appendix 1. For extensive review we refer to the existing literature9.
If a patient is randomised to QFR, the angiographic runs are transferred to the QFR workstation and analysis is performed for all study lesions. QFR (Figure 3) is computed on a Windows-based computer (Medis Suite QAngio XA-3D/QFR solution v2.0; Medis Medical Imaging Systems BV). Only trained, certified observers are allowed to perform QFR study procedures. QFR analysis is performed in accordance with the study-specific standard operating procedure (SOP) (Supplementary Appendix 2). Two end-diastolic frames are selected and vessel contours merged for the 3D reconstruction of the segmented vessel. Long segmentations are recommended to make the contrast frame count more reliable. The contrast frame count is performed in an angiographic run with contrast movement clearly visualised. Virtual QFR pullback values may be used to inform about individual contributions in serial stenoses.
The QFR cut-off for the identification of a flow-limiting stenosis is ≤0.80 for all lesions.
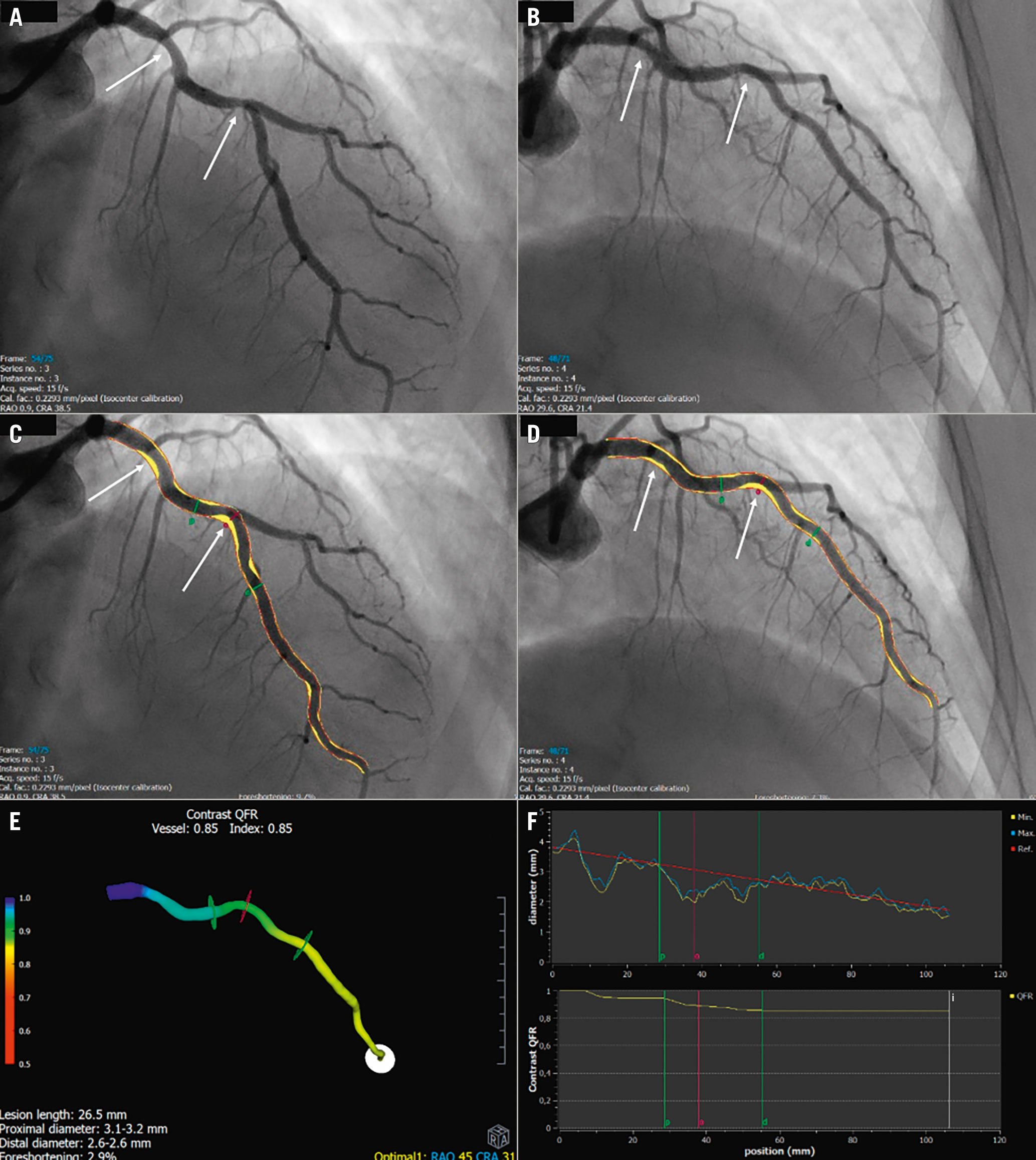
Figure 3. Representative quantitative flow ratio (QFR) analysis. A,B) Two good angiographic projections of the left anterior descending artery (LAD) at least 25° apart. Two solitary stenoses are identified (white arrows). C,D) Vessel is segmented. Plaque is visualised as yellow areas. Red contours represent the reference vessel function. The red “o” indicates where the lesion is causing the largest drop in QFR value. Proximal (p) and distal (d) lesion delimiters are shown in green. E) 3D model of the vessel with colour-coding of pressure distribution. F) Upper panel: diameter graph. The lesion causing the largest pressure drop is marked with “o” and proximal and distal lesion delimiters “p” and “d”. Lower panel: The QFR curve illustrates the decrease in QFR in relation to the two stenoses. The white “i” is an index marker for reading the QFR value at a specific segment allowing for virtual QFR pullbacks.
FFR allocation
Standardised FFR measurements are performed according to current clinical recommendations15. The FFR cut-off for the identification of a flow-limiting stenosis is ≤0.80 for all lesions. See Supplementary Appendix 1 for details.
Coronary revascularisation
Complete coronary revascularisation is attempted by either PCI or CABG. PCI is performed according to current guidelines using CE-marked permanent drug-eluting stents1. Similarly, CABG is performed according to current best practice. QFR should be considered equal to FFR by the surgeon when planning and performing the revascularisation.
Procedure training
QFR observers
The participating sites are requested to allocate staff for training in QFR analysis before performing study procedures. QFR observers are trained and certified by Medis Medical Imaging BV, Leiden, the Netherlands. After the QFR training, all observers are required to submit at least 15 completed QFR analyses for evaluation and feedback from the QFR core laboratory at Aarhus University Hospital. Only fully trained and approved QFR observers can perform study QFR analysis.
Treating physicians
Physicians performing study procedures must complete study-specific training on angiographic quality, as QFR analysis depends on good angiographic quality.
Continuous feedback
At the time of conceptualisation, it was unknown whether a learning curve could persist beyond the mandatory training in QFR analysis. To ascertain a high quality QFR analysis, a systematic feedback on image acquisition quality and QFR analysis is provided on a next day basis for all cases analysed by QFR. Feedback is given in interactive online sessions, as recorded feedback or by standardised written feedback formulas.
Sample size calculation
Using the assumptions of a 5% risk of type 1 error, a 15% risk of type 2 error, an estimated 1-year rate of the primary endpoint in both the FFR and the QFR groups of 6.7%, and a non-inferiority limit of 3.4%, sample sizes of 777 patients in each group (1,554 patients in total) are required to be 85% sure that the lower limit of a 1-sided 95% confidence interval (or an equivalent 2-sided 90% confidence interval) will exclude a difference in favour of the FFR group of more than 3.4%. To accommodate for uncertainty of the population risk and patients lost to follow-up, a total of 2,000 patients will be included.
The non-inferiority limit is determined according to the limits applied in the iFR-SWEDEHEART trial (3.2%) and the DEFINE-FLAIR trial (3.4%), as well as the estimated 1-year rate of the primary endpoint, as a similar population risk is expected. Event rates at 12 months were 6.4% and 6.9% in iFR-SWEDEHEART and DEFINE-FLAIR, respectively23. An absolute margin is applied, as the population mix is expected to be similar to that in the iFR trials.
Statistical analysis
All principal analyses are performed in the intention-to-treat population regardless of the actual diagnostic method performed and treatment received. Analyses in the per-protocol population are performed as sensitivity analyses.
Follow-up begins at randomisation. Analysis for the primary endpoint is assessed by 12-month Kaplan-Meier estimates and compared by unadjusted Cox regression analysis. Further information on statistical analysis is found in Supplementary Appendix 1.
Discussion
Pressure wire-based functional lesion assessment is the established standard for invasive identification of flow-limiting intermediate coronary artery stenosis1. The clinical adoption of FFR is improving but remains low716. A resting index, iFR, was recently implemented in the revascularisation guidelines of the European Society of Cardiology1 based on the results of 2 large non-inferiority clinical outcome trials comparing iFR with FFR23. However, initial data from Italy failed to document an increased use of functional evaluation7. A positive evaluation of QFR would allow for the expansion of functional lesion evaluation. Still, physicians’ confidence in visual assessment could remain an obstacle to a more widespread introduction of functional lesion evaluation.
QFR was first presented in 2016 and has since been extensively validated in paired analysis with FFR as a reference standard. A good numerical agreement between QFR and FFR was demonstrated in a pooled analysis of prospective studies, while some heterogeneity was observed for binary diagnostic performance estimates17. It is anticipated that part of the classification disagreement is an inherent consequence of variability around the diagnostic cut-offs for both tests, as expected for any diagnostic test with a dichotomous cut-off18. Classification mismatch is therefore more frequent in studies with a high fraction of cases approaching the diagnostic cut-off19. Other factors that may have led to classification mismatches include clinical and procedural characteristics such as microvascular dysfunction, previous myocardial infarction, diabetes, or lesion severity172021. However, as feasibility is high and the diagnostic accuracy of QFR appears to be at least as good as iFR with FFR as a reference standard22, it is justified to proceed with a randomised, clinical outcome trial.
The FAVOR III Europe Japan trial is an open-label, randomised trial aimed to show the non-inferiority of QFR compared with FFR. The design is rather similar to the 2 major trials comparing iFR and FFR23. The similarity also includes the non-inferiority limit that was accepted with the 2 previous trials.
The primary endpoint of all-cause mortality, any myocardial infarction and any unplanned revascularisation allows for full characterisation of the new QFR strategy. It was important in the selection of this endpoint over target lesion failure to limit the effect of a potential bias in lesion selection and for assessing the feasibility of QFR as a strategy. Physicians are obliged to assess all intermediate lesions in larger vessels to avoid bias in picking lesions most suitable for QFR or FFR. Thus, the primary endpoint implies that it is important to aim for complete revascularisation based on the diagnostic results. Furthermore, the characterisation of QFR as a strategy allows for evaluation of the potential differences in the number of assessed lesions, the number of revascularised lesions and procedure time. As the primary endpoint includes any unplanned revascularisation, documentation for ischaemia, e.g., by FFR, is required in stable patients at re-evaluation to reduce the risk of bias.
It was claimed that the true difference in outcomes between iFR and FFR was only explored when investigating the subgroup of patients where the 2 tests disagreed23. The FAVOR III trial aims to evaluate whether a strategy based on QFR is non-inferior to a strategy based on FFR with regard to clinical outcomes. Evaluation of a new diagnostic modality requires evaluation of feasibility in clinical practice, e.g., it is unknown if a QFR strategy inadvertently affects the decision to evaluate a lesion or influences the quality of subsequent revascularisation. Thus, a simple lesion-level randomisation or an evaluation of cases with FFR-QFR mismatch may not provide sufficient evidence to support mainstream clinical use.
The study population risk is expected to reflect the risk in the general European population with symptoms suggestive of obstructive coronary artery disease and at least 1 intermediate stenosis. The risk reported in the iFR trials showed a Northern European population with a low incidence of diabetes and low smoking rates. With almost half of the participating sites located in Southern Europe, the population risk in FAVOR III might be slightly higher. Still, we maintain the same non-inferiority limit as applied in the iFR trials. It is currently unknown whether QFR as a strategy results in more repeat revascularisations, and therefore whether QFR will prove non-inferior is not a pre-given fact.
The use of functional coronary lesion evaluation is limited in most parts of the world. With angiographic evaluation still being the standard in several countries, the parallel FAVOR III China trial (ClinicalTrials.gov: NCT03656848) is aimed to show if QFR guidance provides a superior clinical outcome compared with angiographic guidance. The results of both FAVOR III trials could introduce QFR into general clinical practice and thereby improve the diagnostic approach in centres currently relying on angiography alone or could reduce costs related to pressure wire-based functional evaluation.
Limitations
Several design decisions and limitations are discussed above. Additional limitations include 1) risk of biased case selection, as the angiogram could be known before the study procedure, 2) bias in treatment, e.g., if the choice of modus for revascularisation or optimisation with intravascular imaging differs between the groups, and 3) the evaluation before repeat revascularisation with FFR in both groups could favour FFR.
Conclusions
The FAVOR III Europe Japan randomised trial may determine if QFR-guided coronary artery revascularisation is non-inferior to FFR-guided coronary revascularisation with respect to clinical outcomes.
Acknowledgements
We thank data managers Jakob Hjort and Martin Amadeus Rahbek and statistician Lone Juul Hune Mogensen, Aarhus University, Denmark for their important contributions. J. Westra acknowledges support from Aarhus University (PhD scholarship).
Funding
Medis Medical Imaging BV has provided an institutional research grant to fund the trial.
Conflict of interest statement
S. Tu has received institutional research grants from Pulse Medical Imaging. J. Escaned has received consultant fees or speaker fees at educational events from Abbott, Boston Scientific, and Philips/Volcano. U. Landmesser has received lecture or advisory honoraria from Abbott, Amgen, Novarti, Bayer, and Sanofi. G. Campo has received an institutional research grant from Medis Medical Imaging BV. W. Wijns has received an institutional research grant and honoraria from MicroPort; is a co-founder of Argonauts; and an innovation facilitator and medical advisor for Rede Optimus. N.R. Holm received speaker fees and institutional research grants from Abbott, Boston Scientific, and Medis Medical Imaging BV. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.




