Abstract
Messenger RNA (mRNA) is an emerging class of therapeutic agent for the prevention and treatment of a wide range of diseases. The recent success of the two highly efficacious mRNA vaccines produced by Moderna and Pfizer–BioNTech to protect against COVID-19 highlights the huge potential of mRNA technology for revolutionizing life science and medical research. Challenges related to mRNA stability and immunogenicity, as well as in vivo delivery and the ability to cross multiple biological barriers, have been largely addressed by recent progress in mRNA engineering and delivery. In this Review, we present the latest advances and innovations in the growing field of mRNA nanomedicine, in the context of ongoing clinical translation and future directions to improve clinical efficacy.
Similar content being viewed by others
Main
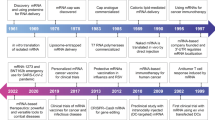
Messenger RNA (mRNA) is a transient carrier that transfers genetic information from DNA to ribosomes, where that information can be translated into proteins1. By delivering mRNAs that express antigens of infectious diseases or cancers, gene-editing components, or disease-related therapeutic proteins, various clinical applications—including vaccines and gene-editing and protein therapies—can be achieved2,3,4. In 1976, it was shown for the first time that nucleic acids could be encapsulated and delivered by tiny particles, in this case composed of polymers5. Although initially ridiculed by the scientific community6, 2 years later, exogenous nucleic acids (this time in the form of mRNAs) were delivered by liposomes and reported to be able to produce proteins in human and mouse cells7,8. Since then, mRNAs have demonstrated therapeutic efficacy in various preclinical studies, laying the foundation for establishing mRNA as a drug and a vaccine9,10.
Nevertheless, the path to the successful application of mRNA as a drug was not straightforward. Initially, the instability, immunogenicity and high production cost of mRNA substantially dampened the enthusiasm of companies and the scientific community for investing resources. Encouragingly, these issues have been gradually addressed by the rapid development of mRNA engineering technologies, including chemical modification11, sequence optimization12,13 and purification14, which drew on the work of numerous researchers over several decades15 (Box 1). These advances laid the foundation for the therapeutic use of mRNA, but clinical translation requires expression in target cells or tissues in vivo. Taking advantage of progress in drug delivery systems6, a wide range of materials16,17—such as lipid nanoparticles (LNPs)18, polymeric nanoparticles19,20 and lipid–polymer hybrid nanoparticles21,22,23—have been developed for in vivo delivery, with the goal of protecting mRNA from rapid degradation by ubiquitous RNases and helping it cross multiple biological barriers9,10,24. Although such technological progress has enabled many preclinical and clinical studies of mRNA drugs over the past two decades, no mRNA nanomedicines (mRNA vaccines or therapies) had been approved by any regulatory authority until very recently, in 2021.
Since then, successes in the development of the two coronavirus 2019 (COVID-19) mRNA vaccines (Moderna mRNA-1273 and Pfizer/BioNTech BNT162b2)25,26,27,28,29 has fueled a renewed and intense research interest in mRNA engineering and delivery, offering the promise of clinical translation of various mRNA-based therapies. Here, we provide an overview of the field of mRNA nanomedicines. We discuss the technical challenges of mRNA-based therapies and link these to biological mechanisms and clinical outcomes. In addition, we highlight the recent innovations and advances in mRNA engineering and delivery methods that have expedited the clinical translation of mRNA therapies for various disorders, including infectious diseases, cancers and inherited diseases.
Challenges regarding the clinical use of mRNA
The clinical use of mRNA for therapeutic purposes requires sufficient mRNA translation in the cells of interest without causing unwanted immune responses. However, achieving this goal requires overcoming several barriers involving mRNA synthesis and delivery within extracellular and intracellular contexts9,24. Therapeutic mRNA is synthesized via in vitro transcription (IVT) in a cell-free system using a linear DNA template generated from a plasmid or PCR and RNA polymerase T7, T3 or SP6 (ref. 30) (Fig. 1a). mRNA is then purified using conventional laboratory-scale purification methods for nucleic acids (for example, precipitation in ethanol). However, these methods often fail to remove impurities such as double-stranded RNA and RNA fragments, which reduce the therapeutic efficacy and cause undesired biological responses in clinical use31. Upon local or systemic administration, mRNA can be rapidly degraded by the abundant nucleases in the extracellular space, removed by macrophage phagocytosis or cleared by renal filtration24,32,33 (Fig. 1b). In the meantime, mRNA is a large, very negatively charged, single-stranded polynucleotide that is difficult to pass through negatively charged cell membranes. In fact, only 0.01% of extravasated mRNAs from blood vessels can enter target cells2, where most of the mRNAs are trapped in endosomes and degraded thereafter (Fig. 1c). Eventually, a fraction of the internalized mRNAs escape from endosomes and reach ribosomes for therapeutic protein translation.
a, Therapeutic mRNA is synthesized in vitro using a linear DNA template and RNA polymerase (T7), followed by purification; it contains a 5′ cap, a 5′ UTR, an ORF encoding the protein of interest, a 3′ UTR and a poly(A) tail. b, After local or systemic delivery, mRNAs face several extracellular challenges, including rapid degradation by the abundant nucleases in the extracellular space, removal by macrophage phagocytosis and clearance by renal filtration. c, A fraction of extravasated mRNAs from blood vessels can be internalized by cells. Most of these internalized mRNAs are trapped in endosomes and can be detected by endosomal and cytosolic RNA sensors, which eventually reduces the translation and stability of the mRNA. An optimized 5′ cap can improve the binding efficacy of cytoplasmic mRNAs to ribosomes, eventually increasing the translation efficacy of mRNA. The endosomal escape of naked and unmodified mRNA is challenging, but can be enhanced by using mRNA carriers. Endosomal RNA sensors include TLR3 (ref. 208), TLR7 (ref. 209) and TLR8 (refs. 210,211). Cytosolic RNA sensors include nucleotide-binding oligomerization domain-containing protein 2 (NOD2)18, melanoma differentiation-associated protein 5 (MDA5)212, retinoic acid-inducible gene-I (RIG-I)213,214 and nucleotide-binding domain leucine-rich repeat-containing family pyrin domain-containing 3 (NLRP3)18. dsRNA, double-stranded RNA; RBCs, red blood cells; ssRNA, single-stranded RNA.
The immunostimulatory potential of exogenous mRNA is another major hurdle to clinical translation15,18 (Fig. 1c). Exogenous mRNA can be sensed by pattern recognition receptors (PRRs) that play a crucial role in responding to viral RNAs, inducing immune stimulation34. Upon endocytosis, mRNAs can be detected by endosomal sensors called Toll-like receptors (TLRs)35, the main family of PRRs expressed primarily but not exclusively by immune cells. mRNAs that escape endosomes can be sensed by several cytosolic PRRs. Stimulation of these PRRs eventually results in the production of type I interferons (IFNs) and other proinflammatory cytokines36. These secreted IFNs bind to their receptors on the stimulated cell and adjacent cells—activating the JAK–STAT pathway, which triggers the transcription of over 300 IFN-stimulated genes. Among these, IFN-inducible protein kinase RNA (PKR) can suppress activity of the translation initiator initiation factor 2, leading to inhibition of mRNA translation37,38, and the 2′-5′-oligoadenylate synthetases39 and RNA-specific adenosine deaminase40 can reduce the stability of mRNAs. Because all of these challenges have substantially limited the clinical use of mRNA, advances in mRNA-related technologies are needed to address these challenges before the full therapeutic potential of mRNAs can be unleashed.
Design of mRNAs and their delivery vehicles
Rapid advances in the field of mRNA engineering and non-viral mRNA delivery have provided various solutions to challenges regarding the clinical use of mRNA. For example, issues with mRNA translatability, stability and immunostimulation can be solved by introducing innovative mRNA designs. Moreover, mRNA delivery vehicles can address at least some of the challenges of mRNA delivery. The rational design of mRNA delivery vehicles requires that they protect mRNAs from degradation by nucleases, cross various biological barriers and efficiently deliver mRNAs into the cytoplasm for robust protein expression.
Design of mRNAs
IVT mRNAs are structurally similar to naturally occurring mature eukaryotic mRNAs, which consist of five major domains: a 5′ cap, a 5′ untranslated region (UTR), an open reading frame (ORF) encoding the protein of interest, a 3′ UTR and a poly(A) tail. The translation and stability of mRNAs can benefit from UTR optimization. UTR sequences from highly expressed genes, such as human β-globin41, are widely used for mRNA synthesis since mRNAs containing these UTRs normally show high levels of translation and stability. Furthermore, improved expression of mRNAs can be achieved by identifying novel UTR sequences using high-throughput screening methods42,43 or deep learning approaches44. Although many 5′ or 3′ UTRs can independently enhance mRNA translation, a rational combination of 5′ and 3′ UTRs can maximize the translation efficiency45. Also, poly(A) tails with lengths of 100–150 nucleotides can improve the stability of mRNAs and efficiently initiate translation by forming complexes with poly(A) binding proteins46,47,48.
One of the most effective strategies to abrogate immunostimulation by IVT mRNAs is nucleoside modification. Compared with unmodified mRNAs, the incorporation of naturally occurring modified nucleosides—such as pseudouridine (ψ), 5‑methylcytidine, N6‑methyladenosine, 5‑methyluridine and 2‑thiouridine—reduces cytokine production11,49,50 by preventing recognition by human TLRs. Mechanistically, the improved translation and stability of ψ-incorporated mRNAs are ascribed to the decreased activation of PKR38 and 2′-5′-oligoadenylate synthetases51. Instead of replacing the original nucleosides with some types of modified nucleosides, simultaneously replacing partial nucleosides with 2‑thiouridine and 5‑methylcytidine substantially suppresses the activation of TLRs and RIG-1 (another PRR) both in vitro and in vivo52. Furthermore, the modified nucleoside N1-methylpseudouridine53 has been shown to have lower cytotoxicity and immunostimulation capacity54 compared with ψ. It is worth noting that both mRNA vaccines from Moderna and Pfizer–BioNTech use nucleoside-modified mRNAs to avoid unintended immune responses. Another strategy to reduce immunostimulation by IVT mRNAs is enhanced mRNA purification. IVT mRNAs purified by high-performance liquid chromatography, free of double-stranded RNA contaminants, display 10- to 1,000-fold higher protein expression in primary cells than unpurified mRNA, without inducing the production of IFNs or inflammatory cytokines14. While high-performance liquid chromatography purification is widely used for mRNA production, a simple, fast and cost-effective cellulose-based purification method provides an alternative for the production of highly pure IVT mRNA55.
The 5′ cap design provides another method to reduce unwanted immunological responses elicited by mRNAs. The natural eukaryotic 5′ cap (cap-0) is a 7-methylguaniosine (m7G) linked to the first nucleotide located at the 5′ end of mRNA through a 5′-5′-triphosphate bridge (m7GpppN)56. Cap-0 sterically inhibits the degradation of mRNAs by nucleases and initiates translation via binding to eukaryotic translation initiation factor 4E56. Compared with cap-0, two additional 5′ caps (cap-1 and cap-2), bearing an additional methyl group on the 2′ hydroxyl of the ribose from the first and second nucleotide, are more widely used in mRNA synthesis due to their lower immunostimulatory potential57. Currently, mRNAs with cap-1 structure can be conveniently manufactured using a co-transcriptional capping method (https://www.trilinkbiotech.com/cleancap), exhibiting minimal immunostimulation and satisfying translation efficiency. In addition, the stability and translation of mRNAs can be simultaneously enhanced by a computational experimental platform58, while translation and immunostimulation can be modulated by chemo-enzymatic modifications59,60.
Messenger RNA delivery vehicles
The rapid clinical translation of mRNA-based vaccines or therapies has benefited from the development of delivery vehicles to protect and deliver the highly unstable mRNA molecules. Currently, the major mRNA delivery systems are lipid-based nanoparticles, polymer-based nanoparticles and lipid–polymer hybrid nanoparticles.
Lipid-based nanoparticles
Lipid-based nanoparticles are the most intensively studied and clinically advanced vehicles for mRNA delivery3,9,61. The most widely used are cationic and ionizable LNPs62, which typically contain a cationic or ionizable lipid, cholesterol, a helper phospholipid and a PEGylated lipid (Fig. 2a). Cationic lipids, such as DOTMA63 or DOTAP64, bearing quaternary ammonium groups, remain positively charged in a pH-independent manner. This cationic environment allows efficient condensation of negatively charged mRNAs, making cationic lipid-based systems the most widely used systems for mRNA delivery in early clinical studies65. Although cationic lipid-based nanoparticles encoding shared tumor antigens or disease-related autoantigens have shown promise in cancer immunotherapy66 and the treatment of experimental autoimmune encephalomyelitis67, their potential cytotoxicity68 and relatively short blood circulation time69 have impeded their clinical translation. To address these issues, lipid-PEGs were employed and a variety of novel ionizable lipids were developed. Unlike cationic lipids, which possess permanent positive charges, ionizable lipids remain neutral at physiological pH but can be protonated at acidic pH70. The neutrality of ionizable lipids in physiological fluids reduces the toxicity and, to some extent, increases the circulation half-life of ionizable LNPs. In addition, the protonation of ionizable lipids at acidic pH not only allows convenient condensation and encapsulation of mRNAs in acidic buffer, but also facilitates the escape of mRNAs from the acidic endosomes48. The PEG layer introduced by lipid-PEGs largely improves the circulation half-life of ionizable lipid-based nanoparticles and reduces nanoparticle aggregation, as well as reducing unfavorable interactions with serum proteins71.
a, LNPs often consist of four basic components: a PEGylated lipid, a helper lipid, cholesterol and a cationic or ionizable lipid. The ionizable lipids largely determine the functions and efficacy of the LNPs. DSPC, distearoylphosphatidylcholine. PEG-DMG, 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol. b, Lipid–polymer hybrid nanoparticles normally contain a cationic or ionizable lipid, a PEGylated lipid and a helper polymer. In some cases, the cationic or ionizable lipid and the helper polymer are replaced by a helper lipid and a cationic polymer. PDSA, poly(disulfide amide). PEG-DSPE, 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene glycol); PEG-DMPE, 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene glycol). c, Polymeric nanoparticles consist simply of cationic polymers.
The development of ionizable lipid-based nanoparticles for mRNA delivery has, to a great extent, benefited from studies over decades on ionizable lipid-based nanoparticles for the delivery of small interfering RNAs (siRNAs), which enable targeted silencing of endogenous mRNAs. For instance, DLin-MC3-DMA (MC3)72,73 is an ionizable lipid designed for the first US Food and Drug Administration–approved siRNA drug patisiran74, which treats hereditary transthyretin-mediated amyloidosis. By optimizing the parameters of these formulations, such as the ratio of RNA to total lipid and the ratio of the aqueous solution to organic solvent (ethanol), MC3-based LNPs have been used in the development of various mRNA-based therapies, including vaccines against Zika virus75,76,77, human immunodeficiency virus78 and Lyme disease79, as well as treatments for cystic fibrosis80 and lymphedema81. One limitation of MC3 is its poor degradability, which can result in toxicity issues when repeated dosing is required. To address this challenge, as well as further increase the efficacy of MC3, Moderna has developed a biodegradable lipid named lipid 5 (ref. 82), which contains primary esters and more branching tails than MC3. Indeed, repeated systemic and local administration of lipid 5-based mRNA LNPs has alleviated acute intermittent porphyria83 and achieved durable anticancer immunity84 in animal models without any obvious toxicity.
In addition to MC3, several other ionizable lipids initially developed for siRNA delivery, such as cKK-E12 (refs. 85,86) and C12-200 (ref. 87), have also been reformulated to deliver mRNAs to the liver for gene editing and protein replacement. However, delivery of therapeutic mRNAs to non-liver tissues by these LNPs is difficult owing to their selective accumulation in livers—a phenomenon probably determined by the ionizable properties of these lipids. Meanwhile, obvious liver toxicity has been observed when cKK-E12-based LNPs have been administered at an mRNA dose of 2.25 mg kg−1 or higher88. Intensive exploration of the new generation of ionizable lipids eventually led to the creation of lipid H/SM-102 (refs. 89,90) and lipid ALC-0315 (ref. 91), resulting in the rapid development of the two effective COVID-19 mRNA vaccines. The favorable safety profiles of these vaccines are probably attributed to the biodegradability of the lipids. In addition, Intellia has achieved robust and persistent in vivo gene editing in animal models using another biodegradable lipid (LP01)-based LNP carrying Cas9 mRNA and guide RNA92. Compared with non-biodegradable lipids, the biodegradable LP01 has less liver bioaccumulation and fewer safety risks. Although the ionizable lipids undoubtedly play a key role in the activity of LNPs, other components are also important. Helper lipids can promote endosomal escape of LNPs by adjusting their fluidity, consequently enhancing their efficacy93, while cholesterol plays an important role in LNP stability.
Lipid–polymer hybrid nanoparticles
Another type of nanoparticle is the lipid–polymer hybrid nanoparticle, which normally includes an ionizable (or sometimes cationic) lipid, a hydrophobic polymer and a PEGylated lipid22,94,95 (Fig. 2b). These nanoparticles have been used to efficiently restore the tumor suppressor PTEN and suppress tumor growth in multiple mouse models of prostate cancer22. In this platform, a hydrophobic helper polymer poly(lactic-co-glycolic acid) (PLGA) replaces the helper lipid and cholesterol of LNPs. Notably, this platform exhibits excellent serum stability96, which can probably be ascribed to the strong hydrophobic interaction between the polymer and lipids, making it ideal for systemic delivery of therapeutic mRNAs to tumors. Replacing the helper polymer PLGA with a redox-responsive polymer poly(disulfide amide) has resulted in a redox-responsive platform for systemic delivery of the tumor suppressor p53 mRNA, achieving efficient tumor suppression in preclinical models21. The PEGylated lipid PEG-DMPE has also been added to improve the efficacy of the platform. Moreover, by simply changing or functionalizing the terminals of lipid-PEGs coated on the surface of the polymeric core, local delivery or organ-specific delivery could easily be achieved. For example, by using DSPE-PEG-NH2 or DSPE-PEG-SH, mucoadhesive mRNA nanoparticles can be generated for intravesical delivery of mRNAs to upregulate desirable proteins in mouse bladder tissues in situ23, and could also be used to upregulate target protein for various other applications within mucosal organs in situ. Other lipid–polymer hybrid nanoparticles have also been recently developed for effective mRNA delivery and tested in preclinical models97,98,99,100.
Polymer-based nanoparticles
Polymeric nanoparticles consist simply of cationic polymers (Fig. 2c). Early studies focused on the use of polyethylenimine or poly-l-lysine for nucleic acid delivery. However, the notable toxicity of polyethylenimine and poly-l-lysine (these highly positively charged polymers can easily interact with negatively charged cellular components, inhibiting normal cellular process) has limited their application in mRNA delivery101. To address this issue, a series of biodegradable poly(β-amino esters) (PBAEs)102,103 have been synthesized. For example, PBAE-based nanoparticles have been used for in vivo delivery of functional mRNAs to circulating T cells104 and multiple tissues105. Furthermore, a hyperbranched PBAE (hPBAE) has been prepared for direct delivery of mRNAs to the lung via inhalation19. Another promising polymer for mRNA delivery is the charge-altering releasable transporter (CART)33,106. Unlike traditional cationic polymers, CARTs can release their mRNA cargo in the cytoplasm through a unique mechanism. The initial positive charges of the oligo(α-amino ester) can effectively condense and encapsulate mRNA and deliver it to the cells, through which the CARTs undergo a degradative, charge-neutralizing intramolecular rearrangement, leading to the rapid release of functional mRNAs. This unique characteristic has resulted in the use of CARTs for in vivo delivery of mRNA to lymphocytes107, which could enable treatment strategies for a wide variety of diseases—for example, effectively stimulating immune responses against tumors108 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV‑2)109.
Promising recent innovations
Innovations in mRNA engineering and delivery have been fueled by the successful development of the COVID-19 mRNA vaccines. These new technologies may potentially produce next-generation mRNAs with greater stability and more robust expression, which could enable the application of mRNAs to the fields of protein therapy and gene editing (usually requiring higher and sustained mRNA expression than mRNA vaccines). In addition, innovations in mRNA delivery can greatly boost the in vivo delivery efficiency of mRNAs in various applications. These innovations should further promote the clinical translation of different mRNA therapies.
Innovations in mRNA engineering
Self-amplifying mRNA
Self-amplifying mRNA contains an alphavirus-based replicon that can amplify the expression of encoded proteins, and therefore requires a much lower dosage than conventional mRNAs in most applications110,111. The incorporation of additional replicon genes makes the size of self-amplifying mRNAs larger than conventional mRNAs. Thus, the formulations used for conventional mRNAs may need to be further optimized for the larger-size self-amplifying mRNAs111,112. While nucleoside modification is widely used in currently approved or currently investigated mRNA-based therapies, self-amplifying mRNAs cannot contain these modifications because they can interfere with the self-amplification process113. Self-amplifying mRNA-based SARS-CoV-2 vaccines have already demonstrated their ability to induce high neutralizing antibody titers in animals114 and several candidates are currently being tested in clinical trials48. These have the potential to be used at lower doses (1–10 µg) than conventional mRNAs (30–100 µg) in COVID-19 vaccines113.
Circular RNA
As discussed, the stability of mRNAs can be substantially improved via nucleoside modification and optimization of coding and non-coding regions. Alternatively, improved stability can be achieved by circularization. Circular RNAs (circRNAs)—single-stranded RNAs with a closed ring structure generated through backsplicing—are a class of non-coding RNAs with potentially broad biological functions115,116. Recent studies have revealed that the protein-coding function of some circRNAs117,118,119 holds great promise for protein translation applications. The unique closed ring structure enables higher stability of circRNAs compared with linear mRNAs, due to the lack of end motifs required for exonuclease-mediated degradation120. Indeed, a pioneering study showed that a circRNA constructed using a self-splicing intron exhibited robust and stable protein expression in eukaryotic cells121. The self-circularization of the linear RNA precursor eventually results in circRNAs containing an internal ribosomal entry site (IRES) to drive the expression of proteins121,122 (Fig. 3a). Besides the enhanced stability, circRNAs induce far fewer undesirable immune responses than unmodified linear mRNAs since they do not activate RNA sensors such as TLRs and retinoic acid-inducible gene-I123. A circRNA vaccine has elicited a higher level of neutralizing antibodies than a linear mRNA vaccine, demonstrating great protective efficacy against SARS-CoV-2 and its emerging variants in mice and rhesus macaques124. More recently, circRNAs containing five key elements, including vector topology, 5′ and 3′ UTRs, IRESs and synthetic aptamers, were constructed125, and simultaneous optimization of these elements resulted in higher circRNA protein yields125. In addition, several companies are exploring other variations on circRNAs, such as optimized IRESs (Fig. 3b).
a, Self-circularization of the linear RNA precursor is initiated by the circularization ribozyme, resulting in the RNA intermediates. The subsequent RNA splice yields the final highly stable circRNA containing an IRES for driving protein expression. b, An endless RNA developed by Laronde could have long-lasting protein translation by using an optimized IRES. c, The PEG10 virus-like particle platform or PEG10 nanoparticle consists of a PEG10 protein for mRNA packaging and fusogen syncytin A (SYNA) for cell targeting. d, A novel fusogenix proteo-lipid vehicle (PLV) platform comprising neutral lipids and proprietary fusion-associated small transmembrane protein enables direct cytosolic mRNA delivery. e, A SORT nanoparticle platform prepared by adding a supplemental SORT molecule of cationic, anionic or ionizable lipid to a conventional LNP system allows selective delivery of mRNAs to the lung, spleen or liver, respectively.
Innovations in mRNA delivery
Novel mRNA delivery systems
While LNPs are the most clinically advanced and widely used systems for mRNA delivery, many other non-LNP systems also have great potential for mRNA delivery. The retrovirus-like protein PEG10 can selectively bind and promote the vesicular secretion of its own mRNAs. Based on this, a PEG10 virus-like particle (VLP) platform, developed by inserting genes of interest (the DNA template of mRNA) into the Peg10 gene, has realized potent gene editing via delivering gene-editing tools into cells126 (Fig. 3c). One major advantage of the PEG10–VLP platform is its minimal immunostimulation and toxicity, given the fact that the platform is constructed using endogenous human proteins. As discussed, the endosomal escape of mRNAs is a major challenge for mRNA delivery and the use of ionizable lipids can ameliorate this, but an alternative strategy is to directly deliver mRNAs into the cytoplasm. To this end, Entos Pharmaceuticals developed a fusogenix proteo-lipid vehicle platform127 using low-toxicity neutral lipids and proprietary fusion-associated small transmembrane proteins128. The unique fusion-associated small transmembrane proteins can facilitate rapid fusion of proteo-lipid vehicle and cell membrane, enabling direct delivery of cargos (for example, mRNAs) into the cytoplasm (Fig. 3d). Similarly, a pH- and redox-responsive coacervate formed by a phase-separating peptide has achieved direct cytosolic delivery of mRNAs to cells and redox-activated release of mRNAs, bypassing classical endocytic pathways129.
Alongside these new platforms, innovations continue to produce more potent LNPs with multiple functions, including enhanced delivery. LNPs containing heterocyclic lipids, identified using a combinatorial library, not only effectively deliver antigen mRNAs to mouse tumors, but also promote antigen-presenting cell maturation via the stimulator of interferon genes (STING) pathway, synergistically increasing the antitumor efficacy130. The efficacy of LNPs can be improved by introducing either unsaturated lipids131 or alkyne lipids88, while the modification of LNPs with a thiol group23 or bisphosphate group132 enables targeted delivery of mRNAs to mucus or bone. In addition, a one-component ionizable amphiphilic Janus dendrimer has enabled efficient delivery of mRNAs to different organs, holding promise for simplification of the current four-component LNP system133,134.
Biological membrane-based vehicles for mRNA delivery
Biological membrane-based vehicles represent another novel biocompatible platform for mRNA delivery. Distinct types of biological membrane-based systems, including cell membrane vesicles135, bacteria-derived outer-membrane vesicles136 and extracellular vesicles137 (for example, exosomes138), have been employed for in vitro and in vivo delivery of therapeutic mRNAs. As a type of nanoscale extracellular vesicle, exosomes have been widely investigated as carriers for drug delivery139,140. For example, Codiak BioSciences has launched the human trial of an engineered exosome-based therapeutic, named exoSTING, for the treatment of solid tumors (NCT04592484). In one preclinical study, exosome-based mRNA vaccines induced robust immunoglobulin G and secretory IgA responses in mice, which were stronger than those induced by liposome-based vaccines141. Thus, biocompatible exosomes may represent a promising platform for mRNA delivery142. One major challenge of the clinical use of current mRNA LNPs for protein replacement therapies is the potential toxicity caused by repeated administrations during a short period. However, biological vesicles are less immunogenic and toxic than most existing platforms, making them particularly useful for repeated mRNA dosing in clinical trials.
Organ- or cell-specific mRNA delivery
Most nanoparticles preferentially accumulate in the liver after intravenous injection88,143,144. Thus, targeted delivery of mRNAs to non-liver tissues will considerably broaden the applications of mRNA therapies. To this end, a selective organ targeting (SORT) nanoparticle platform has been developed for tissue-specific mRNA delivery145. By adding a supplemental SORT molecule of cationic, anionic or ionizable lipid to the widely used four-component LNP system, selective delivery of mRNAs to mouse lung, spleen or liver (respectively) has been achieved, enabling effective CRISPR–Cas9 gene editing (Fig. 3e). A new version of the SORT nanoparticle platform containing a membrane-destabilizing ionizable phospholipid has further improved mRNA delivery efficiency146. A mechanistic study revealed that the binding of specific proteins to the nanoparticles’ surfaces enables their selective accumulation in different tissues147. It is also worth noting that although development and optimization of new nanoformulations for organ-specific delivery of mRNA via intravenous injection is meaningful, changing the routes of administration can be a more practical solution in some contexts. For instance, alternative routes of administration include intravesical delivery of mRNA nanoparticles to target bladder-specific sites23, oral delivery of mRNA via robotic pills to target gastrointestinal tract sites148, and inhaled delivery of mRNA nanoparticles to target lung-specific sites.
In addition to targeting organs, selective delivery of mRNAs to specific cell types allows for more precise and efficient therapies. One strategy for cell type-specific mRNA delivery is developing LNPs or polymeric nanoparticles with formulations optimized for the specific target cell type. For instance, well-optimized149,150, biomimetic151 and imidazole-based152 LNPs and polymers104,108 have been used for the targeted delivery of mRNAs to T cells for cancer immunotherapy, and selective delivery of mRNAs to leukocytes153 has also been achieved. Another strategy is using cell-specific ligands. To enable targeted delivery of therapeutic mRNAs to Ly6c+ inflammatory leukocytes in mice with inflammatory bowel disease154, an anti-Ly6c targeting ligand was conjugated to the LNPs using a modular targeting platform named ASSET (anchored secondary single-chain variable fragment enabling targeting)155. One advantage of this platform is that the targeting monoclonal antibodies can be conveniently replaced according to different applications. Indeed, in another study, a ligand targeting the factor receptor epidermal growth factor receptor (EGFR) was coated to LNPs using the same ASSET platform156. These EGFR-LNPs selectively delivered Cas9 mRNAs and single guide RNAs (sgRNAs) to disseminated EGFR-expressing ovarian tumors in mice, suppressing tumor growth and increasing the survival rate via efficient CRISPR–Cas9 gene editing. Selective delivery of mRNAs to antigen-specific CD8+ T cells157 or CD4+ T cells158 can be achieved by conjugating antigen or CD4 antibodies to LNPs, respectively. With more research, these organ- or cell-specific mRNA delivery platforms will expand the types of diseases that can be treated by mRNA therapies.
Inhalable, intranasal or oral mRNA delivery
Inhalable delivery allows rapid and selective accumulation of mRNA drugs in the lungs, offering great promise for the treatment of lung-related diseases that have been prevalent since the current COVID-19 pandemic began. Inhaled delivery of mRNAs to the lungs was demonstrated using an hPBAE-based nanoformulation, leading to high luciferase protein expression in the lungs of mice19. A subsequent study using this hPBAE platform has achieved efficient Cas13a mRNA delivery to the lungs of mice and hamsters, resulting in the degradation of influenza RNA and reduction of SARS-CoV-2 replication and infection symptoms159. As another non-invasive administration method, intranasal delivery of vaccines can elicit mucosal immunity against respiratory pathogens, making it a promising administration method for SARS-CoV-2 vaccines113. Indeed, intranasal delivery of SARS-CoV-2 mRNA vaccines protected mice from SARS-CoV-2 infection, as demonstrated by the lower viral titer and less tissue damage in the lungs160. One mouse study suggested that immune responses induced by intranasal delivery of mRNA vaccines were lower than those given by intramuscular delivery; a potential reason for this is that the LNPs used nasally were not specifically designed for this delivery route161. LNP formulations targeting appropriate cell types in the upper respiratory tract may mitigate these problems.
Intramuscular injection is the major administration route for currently approved COVID-19 vaccines, but is limited by the requirement for medical or pharmaceutical staff, which may negatively impact vaccine rollout. Oral delivery provides a promising and attractive alternative for COVID-19 vaccine administration, due to its non-invasiveness, patient-friendly features and the possibility for rapid rollout. Encouragingly, an oral adenovirus type 5 SARS-CoV-2 vaccine has successfully reduced disease severity and transmission in a SARS-CoV-2-infected hamster model, leading to a phase 1 clinical trial (NCT04563702)162. Although oral delivery is a more challenging route for fragile mRNAs, its feasibility has been demonstrated in rodents and pigs using ingestible milli-injector capsules, holding great promise for the development of oral mRNA vaccines163,148. BioNTech and Matinas BioPharma recently announced an exclusive research collaboration to develop potential oral mRNA vaccines using a novel lipid nano-crystal platform164. This lipid nano-crystal—a stable crystalline nanoparticle containing multiple layers—is formed via the interaction of calcium and anionic phospholipids, during which active drug molecules inducing mRNAs can be loaded within the layers165.
Translational and clinical studies of mRNA nanomedicine
The aberrant expression of proteins is characteristic of a wide range of diseases. As mRNA technology rapidly progresses, accurate manipulation of the levels of a specific protein can be easily achieved via intracellular delivery of mRNAs encoding the protein of interest (upregulation) or mRNAs encoding gene-editing components (downregulation), making mRNA nanomedicine a promising and versatile tool for the treatment of various diseases. Currently, a range of mRNA nanomedicines, including vaccines (Table 1) and protein or gene-editing therapies (Table 2), are being intensively investigated in clinical studies166.
Vaccines
The two effective mRNA COVID-19 vaccines were developed and rolled out at an unprecedented speed, potentially saving millions of lives and helping rebuild societies worldwide27. However, mRNA COVID-19 vaccines were not the first mRNA nanomedicines to enter clinical trials; many began clinical trials years earlier but their progress has been relatively slow. The success of the mRNA COVID-19 vaccines has strongly fueled the enthusiasm of both investors and researchers in mRNA nanomedicines, leading to numerous innovations and rapid progress in clinical development. While CureVac’s first unmodified mRNA COVID-19 vaccine (CVnCoV) showed an unsatisfying efficacy of 48% (ref. 167) in a phase 2/3 trial (NCT04652102), their second-generation CV2CoV displayed improved efficacy in preclinical studies168 and is now in phase 1 clinical development (NCT05260437). Arcturus developed a self-replicating RNA-based COVID-19 vaccine that protected mice from SARS-CoV-2 infection with a single dose of 2 µg (ref. 169); the phase 3 trial (NCT05012943) showed that their vaccine has 95% efficacy for the prevention of severe COVID-19 disease and 55% efficacy for preventing symptomatic COVID-19 disease170. Notably, Delta and Omicron variants were dominant during the study.
In addition to COVID-19 vaccines, the development of mRNA vaccines against many other infectious diseases has progressed in recent years. For example, Moderna’s mRNA-1647 vaccine (NCT05085366; encoding cytomegalovirus pentamer complex and glycoprotein B antigens against cytomegalovirus) and mRNA-1345 vaccine (NCT05127434; encoding stabilized prefusion F glycoprotein against the respiratory syncytial virus) are being tested in phase 3 trials. More recently, Moderna’s mRNA-1010 seasonal quadrivalent influenza vaccine, encoding World Health Organization-recommended strains, has entered phase 3 trials, making it the fourth mRNA vaccine from Moderna to reach phase 3 (NCT04956575)171. Besides infectious diseases, cancer is another major target of mRNA vaccines being intensively investigated in clinical trials. The Moderna/Merck mRNA-4157 vaccine for advanced melanoma is being investigated in a phase 2 trial (NCT03897881). This is a personalized mRNA cancer vaccine encoding up to 34 neoantigens identified and designed using next-generation sequencing and workflow automation. Similarly, BioNTech has also launched its BNT111 vaccine for melanoma, which has induced durable objective responses in checkpoint inhibitor-treated patients with melanoma in a phase 1 trial (NCT02410733)172.
Protein therapy and gene editing
mRNA-based protein and gene editing therapy has several potential clinical applications (Table 2). Chimeric antigen receptor T cell (CAR-T cell) therapy has demonstrated great efficacy in the treatment of liquid tumors173,174, but its application to solid tumors has proven challenging—partly due to the lack of available targets. Meanwhile, standard CAR-T cell therapy requires the modification of patients’ T cells outside the body, which is expensive and time consuming. To potentially address these challenges, BioNTech has identified several novel solid tumor antigens and developed a CAR-T cell therapy (BNT211) for solid tumors (NCT04503278), in which an mRNA lipoplex encoding CAR-T target antigens is administered to the patient and produces functional CAR-T cells in vivo. In addition to tumors, mRNA-based CAR-T cell therapy has also shown potential for the treatment of cardiac injuries—by generating transient antifibrotic CAR-T cells in vivo (in mice) using modified mRNAs175.
Gene editing is another important application of mRNA nanomedicine176,177,178 that enables mRNAs to downregulate the levels of a specific protein, similar to the function of siRNAs4,94,95,179. In primates, a single-dose treatment of LNPs loaded with mRNAs encoding a CRISPR adenine base editor achieved almost complete knockdown of PCSK9 in the liver, along with 90 and 60% decreases in blood levels of PCSK9 and low-density lipoprotein cholesterol, respectively. Surprisingly, the effect of this treatment persisted for over 8 months180. Intellia developed a biodegradable LP01 ionizable lipid-based system for Cas9 mRNA and sgRNA delivery, achieving >97% knockdown of serum transthyretin upon a single-dose treatment92. These data have led to the human trial (NCT04601051) of an LNP-based gene-editing drug (named NTLA-2001), in which an 87% reduction in the serum transthyretin protein concentration was achieved after a single dose of 0.3 mg kg−1 (ref. 181). These encouraging data will strongly stimulate the clinical translation of more mRNA-based gene-editing therapies.
Challenges of current mRNA nanomedicine
Despite the success of COVID-19 vaccines, mRNA nanomedicines in development still face several challenges. Further innovations and advances are required to overcome these challenges and speed up the clinical translations of more mRNA nanomedicines.
Safety
MC3 is a potent ionizable lipid used in some ongoing clinical trials. However, several preclinical studies have shown that the MC3-based LNPs were immunostimulatory and induced higher expression of proinflammatory cytokines than other LNPs in mice87,182. In addition, intravenous administration of MC3-based human erythropoietin (hEPO) mRNA LNPs to rats and monkeys has resulted in mild toxicological effects with an mRNA dose level of 0.3 mg kg−1 (ref. 183). In this study, liver injury, variations in white blood cell counts and coagulation parameters were observed in rats, whereas reversible complement activation, splenic necrosis and depletion of lymphocytes were observed in monkeys. Notably, the toxicological effects were ameliorated when decreasing the mRNA dose to a lower therapeutic level (0.03 mg kg−1)183. Off-target effects of mRNA LNP vaccines or therapies can lead to undesired mRNA translation in various cells or organs, making them targets of killing184. Continued optimization of organ- or cell-specific mRNA delivery systems will help to address this issue.
Scattered reports have suggested the possibility of the integration of SARS-CoV-2 RNA185 and mRNA from COVID-19 vaccines186 into the genome of host cells through a LINE1-mediated retro-position mechanism. However, this conclusion has been questioned by others187 and more meticulous studies should be conducted to validate this conclusion. If more future studies do show that engineered mRNA sequences can be integrated into the genome of host cells, specific designs can be applied to current mRNAs to inhibit their retro-transcription.
Clinical trials have shown the favorable safety profiles of the two approved COVID-19 mRNA vaccines, and most local and systemic adverse events are mild to moderate25,26. However, as with most other vaccines, rare cases of anaphylactic reactions have been observed188. In the case of mRNA vaccines, one possible reason for these anaphylactic reactions is that the PEGylated lipids in LNPs can induce allergic reactions due to pre-existing antibodies (present in up to 40% of the population)189. It is worth noting that the PEG dose used in current COVID-19 vaccines is much lower than that in other clinical agents and therapies that are reported to trigger rare anaphylactic reactions189,190, and a recent study showed that a patient with pre-existing anti-PEG antibodies tolerated a COVID-19 mRNA vaccine without anaphylactic reactions being triggered191. Therefore, other underlying factors may be associated with these rare adverse events, and a phase 2 clinical trial (NCT04977479) has recently been launched to investigate allergic reactions to the COVID-19 mRNA vaccines.
It will be important to recognize which parts of the mRNA vaccines are responsible for these adverse events, especially in the context of treating protein deficiency and chronic diseases, where high dose or repeated dosing are required, which could further increase the risk of such events. A better understanding of these mechanisms can lead us to optimized formulations that reduce or replace the unfavorable component, thus lowering the risk of adverse events.
Adjuvanticity
Adjuvanticity can be a limitation or an advantage, depending on the context. Due to enhanced translation efficiency and stability, modified mRNAs that cannot elicit immune responses are widely used in ongoing clinical trials11,113. Thus, the mRNAs themselves within mRNA LNP formulations usually do not act as adjuvants. In contrast, the other part of the formulation, the LNP, is known to have adjuvanticity. Mice immunized with intramuscular injection of LNPs and 10 µg of the recombinant hemagglutinin protein immunogen have produced higher antigen-specific T follicular helper cell and germinal center B cell numbers than mice immunized with hemagglutinin protein alone, demonstrating the adjuvant activity of LNPs192. A recent study has also shown that the LNPs of SARS-CoV-2 mRNA vaccines could act as an adjuvant component that induces robust T follicular helper cell and humoral responses193. Notably, LNP has displayed stronger adjuvant potency than a widely used adjuvant, AddaVax193. While neither of the LNP-based COVID-19 mRNA vaccines contains any adjuvants, the strong cellular and humoral immune responses against SARS-CoV-2 elicited by these vaccines might be partially attributed to the adjuvant effect of the LNP component itself. Besides acting as an adjuvant in vaccines for infectious diseases, LNP has also potentiated the antitumor efficacy of the mRNA cancer vaccine by activating TLR4 signaling194. Although more and more studies have demonstrated the adjuvanticity of LNPs, how to manipulate this adjuvanticity is still challenging. To this end, the mechanism of action of LNP adjuvant and the structure–activity relationship of the lipid components should be investigated.
Conclusions and future directions
mRNA nanomedicines have already shown efficacy as vaccines for the prevention of COVID-19 and reducing the risk of hospitalization and death3,27,28,29. Encouraged by this success, more and more mRNA-based vaccines and therapies are expected to reach clinical translation. However, several crucial goals must be reached before the potential of mRNA nanomedicines is fully realized. Increasing evidence suggests that specific biological pathways may interfere with mRNA delivery or translation195,196. Thus, understanding how biological pathways affect in vivo mRNA delivery and translation can further improve the efficacy of mRNA drugs, while the potential toxicity and immune response raised by mRNAs and their carriers should also be carefully considered.
Ultimately, the quick and efficient implementation of mRNA nanomedicines depends largely on their stability and logistical requirements, which impact real-world implementation and rollout. Therefore, innovations that improve stability will be crucial. In recent studies, a thermostable mRNA vaccine was reported to provide protective efficacy against SARS-CoV-2 in mice197 and entered clinical trials198. Moderna’s next-generation COVID-19 vaccine mRNA-1283 could be stable at 2–5 °C.
New engineering advanaces will facilitate real-world applications of mRNA nanomedicines in myriad ways. For example, novel PLGA microparticles could be a promising platform for mRNA delivery with programable drug release and even enable self-boosting vaccines199,200,201. In addition, microneedle patches, which have demonstrated their safety and immunogenicity as carriers for seasonal influenza202 and SARS-CoV-2 (refs. 203,204) vaccines, could be a convenient and minimally invasive platform for mRNA delivery.
Since the enormous potential of mRNA nanomedicines has already been demonstrated by the unprecedented mRNA COVID-19 vaccines, we expect that continued innovation will lead to new and highly efficient mRNA-based therapies, including vaccines for other non-COVID-19 infectious diseases, cancer immunotherapy, protein therapy, gene-editing-based therapy and potentially many others.
References
Sharp, P. A. The centrality of RNA. Cell 136, 577–580 (2009).
Sahin, U., Kariko, K. & Tureci, O. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Xiao, Y. et al. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem. Soc. Rev. 51, 3828–3845 (2022).
Emiliano, B. et al. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale 14, 4448–4455 (2022).
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976).
Langer, R. Controlling the movement of molecules. Q. Rev. Biophys. 52, e5 (2019).
Ostro, M. J., Giacomoni, D., Lavelle, D. O. N., Paxton, W. & Dray, S. Evidence for translation of rabbit globin mRNA after liposomemediated insertion into a human cell line. Nature 274, 921–923 (1978).
Dimitriadis, G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature 274, 923–924 (1978).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Hajj, K. A. & Whitehead, K. A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Lutz, J. et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2, 29 (2017).
Thess, A. et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 23, 1456–1464 (2015).
Karikó, K., Muramatsu, H., Ludwig, J. & Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 39, e142 (2011).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D.mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Gupta, A., Andresen, J. L., Manan, R. S. & Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 178, 113834 (2021).
Granot, Y. & Peer, D. Delivering the right message: challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—an innate immune system standpoint. Semin. Immunol. 34, 68–77 (2017).
Patel, A. K. et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 31, 1805116 (2019).
Moffett, H. F. et al. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat. Commun. 8, 389 (2017).
Kong, N. et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 11, eaaw1565 (2019).
Islam, M. A. et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2, 850–864 (2018).
Kong, N. et al. Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. Proc. Natl Acad. Sci. USA 119, e2112696119 (2022).
Kowalski, P. S., Rudra, A., Miao, L. & Anderson, D. G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 27, 710–728 (2019).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Huang, X. et al. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. https://doi.org/10.1038/s41565-022-01174-5 (2022).
Tang, Z. et al. Insights from nanotechnology in COVID-19 treatment. Nano Today 36, 101019 (2021).
Tang, Z. et al. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 5, 847–860 (2020).
Pardi, N., Muramatsu, H., Weissman, D. & Karikó, K. in Synthetic Messenger RNA and Cell Metabolism Modulation: Methods and Protocols (ed. Rabinovich, P. M.) 29–42 (Humana Press, 2013).
Rosa, S. S., Prazeres, D. M. F., Azevedo, A. M. & Marques, M. P. C. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine 39, 2190–2200 (2021).
Tsui, N. B., Ng, E. K. & Lo, Y. D. J. C. C. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin. Chem. 48, 1647–1653 (2002).
McKinlay, C. J. et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl Acad. Sci. USA 114, E448–E456 (2017).
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137 (2006).
Lee, B. L. & Barton, G. M. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 24, 360–369 (2014).
Devoldere, J., Dewitte, H., De Smedt, S. C. & Remaut, K. Evading innate immunity in nonviral mRNA delivery: don’t shoot the messenger. Drug Discov. Today 21, 11–25 (2016).
García, M. A., Meurs, E. F. & Esteban, M. The dsRNA protein kinase PKR: virus and cell control. Biochimie 89, 799–811 (2007).
Anderson, B. R. et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 38, 5884–5892 (2010).
Silverman, R. H. Viral encounters with 2′,5′-oligoadenylate synthetase and RNAse l during the interferon antiviral response. J. Virol. 81, 12720–12729 (2007).
George, C. X., John, Lijo & Samuel, C. E. An RNA editor, adenosine deaminase acting on double-stranded RNA (ADAR1). J. Interferon Cytokine Res. 34, 437–446 (2014).
Ross, J. & Sullivan, T. Half-lives of beta and gamma globin messenger RNAs and of protein synthetic capacity in cultured human reticulocytes. Blood 66, 1149–1154 (1985).
Cao, J. et al. High-throughput 5′ UTR engineering for enhanced protein production in non-viral gene therapies. Nat. Commun. 12, 4138 (2021).
Orlandini von Niessen, A. G. et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening. Mol. Ther. 27, 824–836 (2019).
Sample, P. J. et al. Human 5′ UTR design and variant effect prediction from a massively parallel translation assay. Nat. Biotechnol. 37, 803–809 (2019).
Zeng, C. et al. Leveraging mRNA sequences and nanoparticles to deliver SARS-CoV-2 antigens in vivo. Adv. Mater. 32, 2004452 (2020).
Linares-Fernández, S., Lacroix, C., Exposito, J.-Y. & Verrier, B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 26, 311–323 (2020).
Holtkamp, S. et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108, 4009–4017 (2006).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Karikó, K., Muramatsu, H., Keller, J. M. & Weissman, D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 20, 948–953 (2012).
Anderson, B. R. et al. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 39, 9329–9338 (2011).
Kormann, M. S. D. et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 29, 154–157 (2011).
Andries, O. et al. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 217, 337–344 (2015).
Nelson, J. et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 6, eaaz6893 (2020).
Baiersdörfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).
Ramanathan, A., Robb, G. B. & Chan, S.-H. mRNA capping: biological functions and applications. Nucleic Acids Res. 44, 7511–7526 (2016).
Schlake, T., Thess, A., Thran, M. & Jordan, I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. 76, 301–328 (2019).
Leppek, K. et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 13, 1536 (2022).
Van Dülmen, M., Muthmann, N. & Rentmeister, A. Chemo-enzymatic modification of the 5′ cap maintains translation and increases immunogenic properties of mRNA. Angew. Chem. Int. Ed. 60, 13280–13286 (2021).
Bollu, A., Peters, A. & Rentmeister, A. Chemo-enzymatic modification of the 5′ cap to study mRNAs. Acc. Chem. Res. 55, 1249–1261 (2022).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Zhang, Y., Sun, C., Wang, C., Jankovic, K. E. & Dong, Y. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 121, 12181–12277 (2021).
Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA 86, 6077–6081 (1989).
Rejman, J. et al. mRNA transfection of cervical carcinoma and mesenchymal stem cells mediated by cationic carriers. J. Control. Release 147, 385–391 (2010).
Kauffman, K. J., Webber, M. J. & Anderson, D. G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release 240, 227–234 (2016).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Krienke, C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Cui, S. et al. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol. Res. 7, 473–479 (2018).
Xia, Y., Tian, J. & Chen, X. Effect of surface properties on liposomal siRNA delivery. Biomaterials 79, 56–68 (2016).
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
Gref, R. et al. Biodegradable long-circulating polymeric nanospheres. Science 263, 1600–1603 (1994).
Jayaraman, M. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. 124, 8657–8661 (2012).
Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013).
Zhang, X., Goel, V. & Robbie, G. J. Pharmacokinetics of patisiran, the first approved RNA interference therapy in patients with hereditary transthyretin-mediated amyloidosis. J. Clin. Pharmacol. 60, 573–585 (2020).
Richner, J. M. et al. Vaccine mediated protection against Zika virus-induced congenital disease. Cell 170, 273–283.e12 (2017).
Richner, J. M. et al. Modified mRNA vaccines protect against Zika virus infection. Cell 168, 1114–1125.e10 (2017).
Pardi, N. et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251 (2017).
Pardi, N. et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 8, 14630 (2017).
Sajid, A. et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 13, eabj9827 (2021).
Mukherjee, A. et al. Engineered mutant α-ENaC subunit mRNA delivered by lipid nanoparticles reduces amiloride currents in cystic fibrosis-based cell and mice models. Sci. Adv. 6, eabc5911 (2020).
Szőke, D. et al. Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema. Nat. Commun. 12, 3460 (2021).
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).
Jiang, L. et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 24, 1899–1909 (2018).
Hewitt, S. L. et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 11, eaat9143 (2019).
Yin, H. et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 35, 1179–1187 (2017).
Song, C.-Q. et al. Adenine base editing in an adult mouse model of tyrosinaemia. Nat. Biomed. Eng. 4, 125–130 (2020).
Riley, R. S. et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 7, eaba1028 (2021).
Miao, L. et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 11, 2424 (2020).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Moderna. Protocol mRNA-1273-P301. Moderna https://covid19crc.org/wp-content/uploads/2020/09/mRNA-1273-P301-Protocol-2020.pdf (2020).
Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine EUA letter of authorization. https://www.fda.gov/media/144412/download (2020).
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018).
Cheng, X. & Lee, R. J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 99, 129–137 (2016).
Huang, X. et al. Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. 17, 748–780 (2022).
Tao, W. et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 12, eaay1063 (2020).
Zhu, X. et al. Long-circulating siRNA nanoparticles for validating prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl Acad. Sci. USA 112, 7779–7784 (2015).
Kaczmarek, J. C. et al. Systemic delivery of mRNA and DNA to the lung using polymer–lipid nanoparticles. Biomaterials 275, 120966 (2021).
Lin, Y.-X. et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 13, eaba9772 (2021).
Xiao, Y. et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 13, 758 (2022).
Kaczmarek, J. C. et al. Optimization of a degradable polymer–lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 18, 6449–6454 (2018).
Lv, H., Zhang, S., Wang, B., Cui, S. & Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 114, 100–109 (2006).
Akinc, A., Lynn, D. M., Anderson, D. G. & Langer, R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J. Am. Chem. Soc. 125, 5316–5323 (2003).
Green, J. J., Langer, R. & Anderson, D. G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 41, 749–759 (2008).
Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020).
Rui, Y. et al. High-throughput and high-content bioassay enables tuning of polyester nanoparticles for cellular uptake, endosomal escape, and systemic in vivo delivery of mRNA. Sci. Adv. 8, eabk2855 (2022).
Benner, N. L. et al. Oligo(serine ester) charge-altering releasable transporters: organocatalytic ring-opening polymerization and their use for in vitro and in vivo mRNA delivery. J. Am. Chem. Soc. 141, 8416–8421 (2019).
McKinlay, C. J., Benner, N. L., Haabeth, O. A., Waymouth, R. M. & Wender, P. A. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl Acad. Sci. USA 115, E5859–E5866 (2018).
Haabeth, O. A. W. et al. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc. Natl Acad. Sci. USA 115, E9153–E9161 (2018).
Haabeth, O. A. et al. An mRNA SARS-CoV-2 vaccine employing charge-altering releasable transporters with a TLR-9 agonist induces neutralizing antibodies and T cell memory. ACS Cent. Sci. 7, 1191–1204 (2021).
Geall, A. J. et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl Acad. Sci. USA 109, 14604–14609 (2012).
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 28, 117–129 (2021).
Maruggi, G., Zhang, C., Li, J., Ulmer, J. B. & Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 27, 757–772 (2019).
Buschmann, M. D. et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines 9, 65 (2021).
McKay, P. F. et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 11, 3523 (2020).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Li, Z. et al. Exon–intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 22, 256–264 (2015).
Pamudurti, N. R. et al. Translation of circRNAs. Mol. Cell 66, 9–21.e27 (2017).
Legnini, I. et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37.e9 (2017).
Koch, L. Translated circular RNAs. Nat. Rev. Genet. 18, 272–273 (2017).
Enuka, Y. et al. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44, 1370–1383 (2015).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
Kauffman, K. et al. Improved immune cell expression with circular RNA (oRNA) in vivo. American Society of Gene & Cell Therapy (ASGCT) Conference 2022 https://www.ornatx.com/wp-content/uploads/2022/05/ASGCT-Poster-221.pdf (2022).
Wesselhoeft, R. A. et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell 74, 508–520.e4 (2019).
Qu, L. et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185, 1728–1744.e16 (2022).
Chen, R. et al. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01393-0 (2022).
Segel, M. et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 373, 882–889 (2021).
Entos Pharmaceuticals. The challenge: effective nucleic acid delivery. Entos Pharmaceuticals https://www.entospharma.com/fusogenix (2022).
Shmulevitz, M. & Duncan, R. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 19, 902–912 (2000).
Sun, Y. et al. Phase-separating peptides for direct cytosolic delivery and redox-activated release of macromolecular therapeutics. Nat. Chem. 14, 274–283 (2022).
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).
Lee, S. M. et al. A systematic study of unsaturation in lipid nanoparticles leads to improved mRNA transfection in vivo. Angew. Chem. Int. Ed. 60, 5848–5853 (2021).
Xue, L. et al. Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J. Am. Chem. Soc. 144, 9926–9937 (2022).
Zhang, D. et al. Targeted delivery of mRNA with one-component ionizable amphiphilic Janus dendrimers. J. Am. Chem. Soc. 143, 17975–17982 (2021).
Zhang, D. et al. One-component multifunctional sequence-defined ionizable amphiphilic Janus dendrimer delivery systems for mRNA. J. Am. Chem. Soc. 143, 12315–12327 (2021).
Park, J. H. et al. Virus‐mimicking cell membrane‐coated nanoparticles for cytosolic delivery of mRNA. Angew. Chem. Int. Ed. 134, e202113671 (2022).
Li, Y. et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv. Mater. 34, 2109984 (2022).
Zhang, S. et al. Selective encapsulation of therapeutic mRNA in engineered extracellular vesicles by DNA aptamer. Nano Lett. 21, 8563–8570 (2021).
Yang, Z. et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 4, 69–83 (2020).
Keener, A. B. J. N. How extracellular vesicles can enhance drug delivery. Nature 582, S14–S15 (2020).
Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 20, 6–7 (2021).
Popowski, K. D. et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter 5, 2960–2974 (2022).
Popowski, K. D. et al. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell. Vesicle 1, 100002 (2022).
Kim, M. et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 7, eabf4398 (2021).
Paunovska, K. et al. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv. Mater. 31, 1807748 (2019).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Tao, W., & Peppas, N. A. Robotic pills for gastrointestinal-tract-targeted oral mRNA delivery. Matter 5, 775–777 (2022).
Billingsley, M. M. et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 22, 533–542 (2022).
Billingsley, M. M. et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 20, 1578–1589 (2020).
Li, W. et al. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat. Commun. 12, 7264 (2021).
Zhao, X. et al. Imidazole‐based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew. Chem. Int. Ed. 132, 20258–20264 (2020).
Ramishetti, S. et al. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv. Mater. 32, 1906128 (2020).
Veiga, N. et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 9, 4493 (2018).
Kedmi, R. et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 13, 214–219 (2018).
Rosenblum, D. et al. CRISPR–Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 6, eabc9450 (2020).
Su, F.-Y. et al. In vivo mRNA delivery to virus-specific T cells by light-induced ligand exchange of MHC class I antigen-presenting nanoparticles. Sci. Adv. 8, eabm7950 (2022).
Tombácz, I. et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNP. Mol. Ther. 29, 3293–3304 (2021).
Blanchard, E. L. et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Nat. Biotechnol. 39, 717–726 (2021).
Li, J.-Q. et al. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice. Signal Transduct. Target. Ther. 6, 369 (2021).
Anderluzzi, G. et al. The role of nanoparticle format and route of administration on self-amplifying mRNA vaccine potency. J. Control. Release 342, 388–399 (2022).
Langel, S. N. et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 14, eabn6868 (2022).
Abramson, A. et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter 5, 975–987 (2022).
Matinas BioPharma. BioNTech and Matinas BioPharma announce exclusive research collaboration to evaluate novel delivery technology for mRNA-based vaccines. Matinas BioPharma https://www.matinasbiopharma.com/investors/news-events/press-releases/detail/419/biontech-and-matinas-biopharma-announce-exclusive-research (11 April 2022).
Matinas BioPharma. Lipid nano-crystal (LNC)—a disruptive platform for the safe and targeted delivery of therapeutics. Matinas BioPharma https://www.matinasbiopharma.com/lnc-technology/lnc-platform (2022).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Kremsner, P. G. et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 22, 329–340 (2022).
Gebre, M. S. et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 601, 410–414 (2022).
De Alwis, R. et al. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Mol. Ther. 29, 1970–1983 (2021).
Arcturus Therapeutics. Arcturus announces self-amplifying COVID-19 mRNA vaccine candidate ARCT-154 meets primary efficacy endpoint in phase 3 study. Arcturus Therapeutics https://ir.arcturusrx.com/news-releases/news-release-details/arcturus-announces-self-amplifying-covid-19-mrna-vaccine (20 April 2022).
Moderna. Moderna announces first participants dosed in phase 3 study of seasonal influenza vaccine candidate (mRNA-1010). Moderna https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participants-Dosed-in-Phase-3-Study-of-Seasonal-Influenza-Vaccine-Candidate-mRNA-1010/default.aspx (7 June 2022).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Tran, E., Longo, D. L. & Urba, W. J. A milestone for CAR T cells. N. Engl. J. Med. 377, 2593–2596 (2017).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Huang, X. et al. Efficient delivery of mRNA using crosslinked nucleic acid nanogel as a carrier. ACS Mater. Lett. 2, 1509–1515 (2020).
Rothgangl, T. et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 39, 949–957 (2021).
Zhang, D. et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 17, 777–787 (2022).
Huang, X. et al. Intercalation-driven formation of siRNA nanogels for cancer therapy. Nano Lett. 21, 9706–9714 (2021).
Musunuru, K. et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 593, 429–434 (2021).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).
Maugeri, M. et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 10, 4333 (2019).
Sedic, M. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Vet. Pathol. 55, 341–354 (2018).
Igyártó, B. Z., Jacobsen, S. & Ndeupen, S. Future considerations for the mRNA–lipid nanoparticle vaccine platform. Curr. Opin. Virol. 48, 65–72 (2021).
Zhang, L. et al. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl Acad. Sci. USA 118, e2105968118 (2021).
Aldén, M. et al. Intracellular reverse transcription of Pfizer BioNTech COVID-19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Curr. Issues Mol. Biol. 44, 1115–1126 (2022).
Parry, R., Gifford, R. J., Lytras, S., Ray, S. C. & Coin, L. J. M. No evidence of SARS-CoV-2 reverse transcription and integration as the origin of chimeric transcripts in patient tissues. Proc. Natl Acad. Sci. USA 118, e2109066118 (2021).
Shimabukuro, T. T., Cole, M. & Su, J. R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020–January 18, 2021. J. Am. Med. Assoc. 325, 1101–1102 (2021).
Kozma, G. T., Shimizu, T., Ishida, T. & Szebeni, J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 154-155, 163–175 (2020).
Muskula, P. R. & Main, M. L. Safety with echocardiographic contrast agents. Circ. Cardiovasc. Imaging 10, e005459 (2017).
Corey, K. B. et al. A case of coronavirus disease 2019 messenger RNA vaccine tolerance and immune response despite presence of anti-polyethylene glycol antibodies. Ann. Allergy Asthma Immunol. 129, 246–248 (2022).
Pardi, N. et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 215, 1571–1588 (2018).
Alameh, M.-G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e7 (2021).
Zhang, H. et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl Acad. Sci. USA 118, e2005191118 (2021).
Lokugamage, M. P. et al. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv. Mater. 32, 1904905 (2020).
Paunovska, K. et al. Increased PIP3 activity blocks nanoparticle mRNA delivery. Sci. Adv. 6, eaba5672 (2020).
Zhang, N.-N. et al. A thermostable mRNA vaccine against COVID-19. Cell 182, 1271–1283.e16 (2020).
Chen, G.-L. et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Microbe 3, e193–e202 (2022).
McHugh, K. J. et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science 357, 1138–1142 (2017).
Lu, X. et al. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci. Transl. Med. 12, eaaz6606 (2020).
Sarmadi, M. et al. Experimental and computational understanding of pulsatile release mechanism from biodegradable core-shell microparticles. Sci. Adv. 8, eabn5315 (2022).
Rouphael, N. G. et al. Immunologic mechanisms of seasonal influenza vaccination administered by microneedle patch from a randomized phase I trial. NPJ Vaccines 6, 89 (2021).
Xia, D. et al. An ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination. Proc. Natl Acad. Sci. USA 118, e2110817118 (2021).
O’Shea, J., Prausnitz, M. R. & Rouphael, N. Dissolvable microneedle patches to enable increased access to vaccines against SARS-CoV-2 and future pandemic outbreaks. Vaccines 9, 320 (2021).
Moderna. Moderna announces Omicron-containing bivalent booster candidate mRNA-1273.214 demonstrates superior antibody response against Omicron. Moderna https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-Omicron-Containing-Bivalent-Booster-Candidate-mRNA-1273.214-Demonstrates-Superior-Antibody-Response-Against-Omicron/default.aspx (8 June 2022).
Sablad, M. et al. mRNA therapy for ornithine transcarbamylase deficiency. Society for Inherited Metabolic Disorders (SIMD) Conference 2019 https://secureservercdn.net/45.40.151.233/e32.83a.myftpupload.com/wp-content/uploads/2020/09/April-2019-OTC-Poster-Presented-at-SIMD.pdf?time=1654792975 (2019).
Arcturus Therapeutics. LUNAR-OTC. Arcturus Therapeutics https://arcturusrx.com/mrna-medicines-pipeline/#lunarOTC (2022).
Liu, L. et al. Structural basis of Toll-like receptor 3 signaling with double-stranded RNA. Science 320, 379–381 (2008).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Sousa, C. R. E. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Tanji, H. et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22, 109–115 (2015).
Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Züst, R. et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 12, 137–143 (2011).
Schlee, M. et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Cobb, M. Who discovered messenger RNA? Curr. Biol. 25, R526–R532 (2015).
Conry, R. M. et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 55, 1397–1400 (1995).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Acknowledgements
We are supported by the US METAvivor Early Career Investigator Award (2018A020560 to W.T.), Harvard Medical School/Brigham and Women’s Hospital Anesthesia Department Basic Scientist Grant (2420 BPA075 to W.T.), Khoury Innovation Award (2020A003219 to W.T.), Gillian Reny Stepping Strong Center for Trauma Innovation Breakthrough Award (113548 to W.T.), Nanotechnology Foundation (2022A002721 to W.T.), Farokhzad Family Distinguished Chair Foundation (018129 to W.T.) and American Heart Association Collaborative Sciences Award (2018A004190 to W.T.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.L. declares the following current competing interest: Moderna. A list of all competing interests for R.L., past and present, is provided as Supplementary Table 1. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Kong, N., Zhang, X. et al. The landscape of mRNA nanomedicine. Nat Med 28, 2273–2287 (2022). https://doi.org/10.1038/s41591-022-02061-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-02061-1
This article is cited by
-
The quest for nanoparticle-powered vaccines in cancer immunotherapy
Journal of Nanobiotechnology (2024)
-
Tailor made: the art of therapeutic mRNA design
Nature Reviews Drug Discovery (2024)
-
High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models
Nature Communications (2024)
-
tRNA therapeutics for genetic diseases
Nature Reviews Drug Discovery (2024)
-
In situ combinatorial synthesis of degradable branched lipidoids for systemic delivery of mRNA therapeutics and gene editors
Nature Communications (2024)