Abstract
Purpose
Pulsed-field ablation (PFA) is a new energy source to achieve pulmonary vein isolation (PVI) by targeted electroporation of cardiomyocytes. Experimental and controlled clinical trial data suggest good efficacy of PFA-based PVI. We aimed to assess efficacy, safety and follow-up of PFA-based PVI in an early adopter routine care setting.
Methods
Consecutive patients with symptomatic paroxysmal or persistent atrial fibrillation (AF) underwent PVI using the Farawave® PFA ablation catheter in conjunction with three-dimensional mapping at two German high-volume ablation centers. PVI was achieved by applying 8 PFA applications in each PV.
Results
A total of 138 patients undergoing a first PVI (67 ± 12 years, 66% male, 62% persistent AF) were treated. PVI was achieved in all patients by deploying 4563 applications in 546 PVs (8.4 ± 1.0/PV). Disappearance of PV signals after the first application was demonstrated in 544/546 PVs (99.6%). More than eight PFA applications were performed in 29/546 PVs (6%) following adapted catheter positioning or due to reconnection as assessed during remapping. Mean procedure time was 78 ± 22 min including pre- and post PVI high-density voltage mapping. PFA catheter LA dwell-time was 23 ± 9 min. Total fluoroscopy time and dose area product were 16 ± 7 min and 505 [275;747] cGy*cm2. One pericardial tamponade (0.7%), one transient ST-elevation (0.7%) and three groin complications (2.2%) occurred. 1-year follow-up showed freedom of arrhythmia in 90% in patients with paroxysmal AF (n = 47) and 60% in patients with persistent AF (n = 82, p = 0.015).
Conclusions
PFA-based PVI is acutely highly effective and associated with a beneficial safety and low recurrence rate.
Graphical abstract

Similar content being viewed by others
Introduction
Pulmonary vein isolation (PVI) is an important component of rhythm control therapy in patients with atrial fibrillation (AF). Durable and safe isolation of all PVs remains difficult [1] despite technical improvements including introduction of cryoballoon single shot devices [2], irrigated radiofrequency catheters, and contact force sensing ablation catheters [3]. In addition, unintended damage to other atrial or extracardiac structures can cause rare but severe complications, such as phrenic nerve palsy [4], PV stenosis [5], or atrio-esophageal fistula.
Pulsed-field ablation (PFA) is a nonthermal energy source in which electrical fields are used to disrupt the membrane of cardiomyocytes, whereas damage to other intra- and extracardiac anatomical structures, such as vessels, nerves or the esophagus is limited in experimental settings [6,7,8,9]. Known complications of thermal energy sources such as atrio-esophageal fistula, phrenic nerve palsy or PV stenosis have not been observed yet after PFA [10,11,12]. Based on these observations, PFA might have the potential to improve effectiveness of PVI with lower complication rates. Initial clinical observations in controlled trials appear to confirm the efficacy of PFA-based PVI results, showing PV reconnection in 4% of PVs (16% of patients) at 90 days and freedom from any atrial tachycardia in 85% [13], but more clinical information on the safety, efficacy and follow-up data of PFA-based PVI is needed.
Here, we report the periprocedural safety, efficacy and follow-up data of PFA-based PVI in two German high-volume centers.
Methods
Patient population
Data from consecutive patients with symptomatic paroxysmal or persistent AF who underwent PVI using the novel PFA ablation catheter (Farawave®, Farapulse Inc, Menlo Park, CA, USA) were analyzed. Exclusion criteria were prior PVI or left atrial (LA) ablation, LA diameter > 60 mm, severe valvular heart disease or contraindications to post-interventional oral anticoagulation. The study was performed in accordance with the Declaration of Helsinki of 2013, and it was designed as a prospective observational study approved by the ethics committee in Hamburg (NCT05521451, 2020-10066-BO) and Bad Oeynhausen (2019-563).
Preprocedural management
Transesophageal echocardiography or cardiac computed tomography were performed to rule out LA thrombus prior to ablation. In patients on vitamin K antagonists, ablation was performed under therapeutic INR values of 2–3. Novel oral anticoagulants were skipped in the morning of the procedure. All patients provided written informed consent.
Ablation procedure
The procedure was performed under deep sedation or general anesthesia with endotracheal intubation applying propofol, fentanyl and optional midazolam. After femoral venous access, a 7 F steerable decapolar catheter (Inquiry™, Abbott) was positioned in the coronary sinus. Following single transseptal puncture guided by fluoroscopy and using a modified Brockenbrough technique, a SL1 sheath (8.5 F, St. Jude Medical, MN, USA) was advanced into the LA. After transseptal puncture, intravenous heparin was administered to maintain an activated clotting time of >300 s. Selective PV angiography was performed to identify the individual PV ostium. Procedures in Bad Oeynhausen were performed after prior cardiac computer tomography or magnetic resonance imaging for three-dimensional (3D) integration (EnSite™NavX™and MediGuide™, Abbott). A 3D electroanatomical map of the LA was generated using a multipolar mapping catheter in conjunction with CARTO3 (Biosense Webster, Irvine, CA, USA) or the Ensite cardiac mapping system (NavX, Abbott, Inc.). Thereafter, the SL1 sheath was changed over-the-wire for a steerable sheath (Faradrive, 13 F inner and 16.8 F outer diameter). A 12 F multielectrode pentaspline PFA catheter (Farawave, Farapulse) was advanced over a soft, conventional J-tip wire (e.g., InQwire Guidewire J Tip-3 mm × 180 cm, Merit Medical). The PFA catheter size (diameter of 31 vs. 35 mm measured in fully deployed flower configuration) was chosen based on PV visualization by angiography and LA size. Preinterventional injection of 1 mg atropine were applied at operator’s discretion. After two applications in the basket configuration the pentaspline catheter was rotated one tenth of a rotation for another 2 applications in the same configuration. Thereafter 2 plus 2 applications were added in the flower configuration. Left common PVs were isolated by a similar approach receiving 16 applications (8 applications with the guidewire in the upper branch lower branch each). Generator output ranged from 1.8 to 2.0 kV per application during the course of the study with 5 biphasic and bipolar pulsed electrical fields of 200 ms duration each and 300 ms pause (2.5 s per application). PVI was defined as elimination of all PV signals and confirmed by entrance block using a circular mapping catheter. Remapping was performed to assess lesion formation. Other electrophysiological protocols performed after completion of PVI and remapping, e.g., programmed stimulation, slow-pathway modulation, or ablation at the cavo-tricuspid isthmus were not counted for procedure time of PVI.
Endpoints
The primary endpoint was electrical PVI. Secondary endpoints included: PVI after single PFA application, acute PV reconnection, procedure-, LA dwell- and fluoroscopy times, dose area product, freedom from AF, atrial tachycardia or atrial flutter during 1-year follow up following a 90 days blanking period postablation, and procedure-related complications. The following complications were classified as major: pericardial effusion or tamponade, transient ischemic attack of stroke, atrioesophageal fistula, access site complications requiring surgical repair or blood transfusion. The following complications were classified as minor: Access site bleeding not requiring surgical or interventional therapy or blood transfusion.
Postprocedural care
Following ablation all patients underwent transthoracic echocardiography to rule out pericardial effusion. All patients underwent a thorough clinical evaluation after the procedure and on the day of discharge, including a neurological assessment, to capture complications. All patients were treated with proton‐pump inhibitors for 6 weeks. Low molecular‐weight heparin was administered in patients on vitamin K antagonists in case of an INR < 2.0 until a therapeutic INR of 2 to 3 was reached. Novel oral anticoagulants were resumed 6 h post ablation. Anticoagulation was continued for at least 3 months, and thereafter based on the individual CHA2DS2‐VASc score. After the procedure antiarrhythmic drugs were stopped at the discretion of the operator.
Follow-up
Patients were interviewed at 3, 6 and 12 months after the procedure concerning symptomatic recurrences. ECGs and Holter–ECGs were requested when in person follow-up was not conducted at the institution. Any episode of AF, atrial tachycardia (AT) or atrial flutter (AFl) as documented in 12-lead ECG or in Holter ECG > 30 s was considered a recurrence. Episodes that occurred during the first 90 days (blanking period) after the procedure were not considered recurrences.
Statistical analysis
Continuous data are described as means and standard deviations, if the variables are normally distributed, or as medians, 25th and 75th percentiles otherwise. Categorical data are described with absolute and relative frequencies. All p values are two-sided and p < 0.05 is considered to indicate statistical significance. All calculations were performed with GraphPad Prism 6 or Microsoft Excel 365.
Results
Patient characteristics
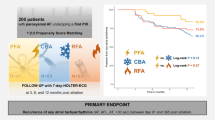
138 Patients with symptomatic paroxysmal (38%) or persistent AF (62%) were analyzed, 101 consecutive patients in Hamburg and 37 consecutive patients in Bad Oeynhausen (Fig. 1). Mean age was 67 ± 12 years with male predominance (66%), CHA2DS2–VASc Score of 2.7 ± 1.7, and with moderate LA enlargement (diameter 43 ± 5 mm, volume index 37 ± 13 ml/m2). Detailed patient characteristics are given in Table 1.
Procedural parameters
Mean procedure time (defined as time from femoral access until sheath removal or begin of a secondary ablation) was 78 ± 22 min including pre- (15 ± 8 min) and post-PVI (11 ± 5 min) voltage mapping time (Fig. 2C). Mean LA dwell time was 60 ± 20 min, PFA catheter dwell time was 24 ± 10 min (Table 2). The mean total number of PFA-applications was 33 ± 3/patient corresponding to a PVI mean ablation time of 83 ± 8 s/patient. Most additional applications were applied at right-sided PVs (23/277) vs. left-sided PVs (6/269; p < 0.001, Fig. 3). During the first 101 cases in Hamburg the PFA catheter dwell time was reduced from 32 ± 10 min (first 30 pts) to 23 ± 8 min (p < 0.001; 61–101st patient, Fig. 4), which was not significantly different from patient 31–61st (24 ± 10 min, p = 0.951). This reflects a learning curve reaching a plateau after approximately 30 procedures.
A Example of pulsed-field ablation catheter visualization in CARTO3 (Biosense Webster) at the LIPV in posterior–anterior (left) and left lateral projection (right). B Example of disappearance of pulmonary vein signals at the left superior pulmonary vein after the first pulsed-field application. C Three-dimensional electroanatomic voltage map of the left atrium in postero-anterior before (left) and after PFA-based pulmonary vein isolation (right)
Quantification of pulsed-field applications additional to the recommended 8 applications per pulmonary vein. Green represents additional application due to positioning on operator’s discretion. Red represents additional applications due to reconnection of pulmonary vein. LSPV = left superior PV, LIPV = left inferior PV, LC = left common, PFA = pulsed-field ablation, PV = pulmonary vein, RIPV = right inferior PV; RSPV = right superior PV
Acute efficacy
Successful electrical PVI was achieved in 546/546 PVs (100%), including 8 left common PVs and 1 left and 1 right middle PV. Of note, PV signals recorded on the pentaspline PFA catheter disappeared after the first application (2.5 s) in 544/546 PVs (99.5%, for example, see Fig. 2B). A total of 515/546 PVs (94.3%) was treated by 8 applications (4 applications each in the basket and flower configuration, Fig. 5) according to the current recommendations. In 17/138 patients (12.3%) and 20/546 PVs (3.7%) additional applications were deployed due to adapted positioning of the catheter at the respective PVs (Table 3). Acute PV reconnection was observed during remapping in 9/138 patients (7%) and in 10/546 PVs (1.8%, Fig. 3, Table 3). All these PVs were re-isolated by additional PFA applications in adapted catheter positions. No RF touch-up was necessary. PFA induced wide antral circumferential lesions around the ipsilateral PVs (Fig. 2), even in left common PVs (S-Fig. 1). In a subset of post-PVI voltage maps (n = 33), posterior distance between left and right low-voltage areas was reduced from 69 ± 12 mm prior ablation to 25 ± 10 mm (p < 0.001) after PVI at superior PVs and from 61 ± 10 mm to 25 ± 10 mm (p < 0.001) at inferior PVs with a remaining non-ablated LA posterior wall area of 11.9 ± 4.7 cm2.
Pulsed-field ablation catheter (FARAPULSE) in its basket (A) and flower (B) configuration. Corresponding fluoroscopic views of the pulsed-field ablation catheter at a right superior pulmonary vein in basket (C, right anterior oblique 30°) and flower (D, left anterior oblique 40°) configuration. Red arrow indicates subequatorial electrode exemplary for one spline, which can be visualized in 3D mapping systems (Fig. 3)
Phrenic capture during PFA pulses was observed in 90/93 (96.8%) patients and was more frequently seen along the right than left PVs (88/93 vs. 30/93, p < 0.001) and more often at the superior vs. inferior PVs (85/93 vs. 75/93, p = 0.035). In detail, phrenic nerve capture occurred at 83/93 RSPVs (89.2%), 71/93 RIPVs (76.3%), 25/93 LSPVs (26.9%) and 15/93 LIPVs (16.1%). Atropine was administered in a total of 88/138 patients to reduce vagally induced bradycardia due to sinus pause (S-Fig. 2) or atrioventricular block (S-Fig. 3). 4/138 patients received atropine after previous distinct vagal reactions following first PFA pulses. Asystole was much shorter in patients with previous atropine administration (2.0 ± 0.7 s, n = 10) than without (5.9 ± 3.3 s, n = 10, p = 0.018). 49/138 patients did not receive atropine due to the operator’s discretion or due to implanted pacemakers with a ventricular lead or due to the ability to pace the ventricle via the coronary sinus catheter.
Integration in mapping system
The PFA pentaspline catheter could be visualized within the 3D map with both mapping systems (CARTO3, Bisosense Webster, Fig. 2 and EnSite™NavX™, Abbott, S-Fig. 1), helping to navigate the catheter. The subequatorial electrode of each spline (third electrode) could be connected to the mapping system (Fig. 5), resulting in a ring in the 3D map (Fig. 2A), representing the external dimension of the catheter in basket or flower configuration. During PFA pulses it is recommended to pause the catheter visualization. When visualization was not paused, the catheter disappeared during PFA pulses and re-appeared within a few seconds afterward, but in few cases 3D mapping matrix was lost. With the Ensite mapping system, the pentaspline PFA catheter could also be used to create a 3D voltage map of the LA recorded by 5-electrodes in total, although mapping resolution was reduced compared to dedicated mapping catheters.
Non-PV applications
Two patients developed an atrial tachycardia during the procedure and according to entrainment maneuvers the lateral anterior wall was involved in the circuit. PFA applications (4 applications in basket configuration in the first patient and 8 applications, 4 in basket- and 4 in flower configuration, in the second patient) at the anterior wall terminated the tachycardias. However, bidirectional conduction block was not assessed. In one patient with persistent AF, the posterior wall was isolated by 4 applications in flower configuration.
One patient with persistent AF suffered from repetitive onset of AF after multiple electrocardioversions after PFA-based PVI. The trigger origin was identified within the superior vena cava (SVC). The PFA catheter was then placed into the SVC and 4 PFA applications were conducted. During these applications sinus arrest occurred and after 5 min of atrial pacing sinus rhythm returned. After SVC ablation AF did not re-occur. However, isolation of SVC was not assessed.
In 4/138 patients (2.9%) bidirectional block of the CTI was performed after PVI with PFA by 9 ± 2 applications and in 9/138 patients (6.5%) by RF ablation. One patient received slow-pathway modulation by RF after induction of atrioventricular nodal reentry tachycardia.
Periprocedural complications
Periprocedural complications were one cardiac tamponade requiring pericardiocentesis (blood gas analysis suggested venous bleeding, potentially indicating an association with a difficult insertion of the CS-catheter in this procedure), one patient experienced transient ST-elevation and concomitant atrioventricular-block for 3 min which resolved spontaneously and three minor groin site complications which were treated conservatively. One patient described minor neurological missperceptions and underwent cerebral MRI without any pathological signs, while symptoms disappeared within hours. No other adverse events occurred.
Follow-up
129 of 137 (94%) patients could be included into follow-up analysis with a mean follow-up time of 249 ± 90 days after PVI. The single procedure Kaplan–Meier estimates for freedom from AF, AFl or AT was 60 ± 10% for patients with persistent AF (n = 82) vs. 90 ± 6% in patients with paroxysmal AF (n = 47, p = 0.015, Mantel–Cox test, Fig. 6). At last follow-up, class I or III antiarrhythmic drug therapy was continued in 2.4% patients with persistent AF and in 2.1% with paroxysmal AF.
Discussion
To the best of our knowledge, this study is the largest multi-center report in routine care evaluating acute efficacy, periprocedural safety and 1-year follow-up of PFA-based PVI system in patients with AF.
Main findings are:
-
1.
PFA-based PVI achieves complete acute isolation of PV (546 of 546 PV),
-
2.
acute complication rates are low (1/138 patients, 0.7%),
-
3.
the PFA catheter can be visualized using commonly used mapping systems,
-
4.
the system provides a short learning curve and a short PFA catheter LA dwell time,
-
5.
atropine reduced the duration of post-PFA asystole and heart block,
-
6.
freedom of AF/AT/AF was 90% for paroxysmal AF and 60% for persistent AF patients during 1-year follow-up.
Efficacy of PFA-based PVI
In the current study we demonstrated that PVI by PFA and the Farawave catheter could be successfully performed in a total of 138 patients and in two independent German high-volume centers. Ablation was characterized by short procedure duration and ultrashort PVI ablation times when compared to reports of established systems, such as RF in conjunction with 3D mapping [4, 14]. In comparison to other PFA single-shot devices integrated in a circular catheter [15], the pentaspline catheter (basket/flower configuration) seems to have shorter procedure and LA dwell times, probably due to facilitated positioning. Total procedure times without 3D mapping would achieve < 60 min in this series. The combination of PFA catheter ablation and different 3D mapping platforms was easy and safe at both centers. Thus, the PFA-based system can be used for PVI [16, 17] including to achieve the clinical benefit of early rhythm control therapy [18] and in view of the superiority of ablation over drug-based treatment strategies regarding freedom from AF [19, 20].
In our “all comer” cohort we did not find any hint that PFA–PVI might be less successful in patients with advanced atriopathy. Nevertheless, similar to other energy-forms of PVI, non-PV trigger might jeopardize clinical success of PVI in patients with advanced atriopathy.
The rather short learning curve regarding PFA catheter LA dwell time demonstrated easy adoption of the technology in daily practice even when performed by multiple operators, reaching mean PFA LA dwell times of 23 min, which is even lower than previously reported for the pentaspline PFA catheter [13].
Comparable to previously published data from Reddy et al.[21], the intraprocedural PVI durability as assessed by final remapping was high and demonstrated in 98.4% PVs. PVs with electrical reconnection could be finally isolated with additional applications in adapted catheter positionings by, e.g., placing the guide wire to another PV branch or by giving the PFA catheter a more anterior orientation in the flower configuration.
Safety
The safety profile with a single pericardial tamponade as the only major complication, and no TIA/stroke or phrenic nerve paralysis is consistent with previously published data [13]. Whether lack of esophageal or neuronal damage is due to the specifics of the PFA energy, or due to chance, will need further evaluation in independent data sets. Nevertheless, access to the LA via an 18 F sheath might bear a risk for air embolism, which might have been the cause of the observed transient ST-elevation and concomitant AV-block.
Follow-up
Clinical success of PVI depends on PV durability and non-PV triggers. PV durability showed large heterogeneity in remapping studies after PVI with thermal energy sources (12–79% of patients) [1] and 84% in one remapping trial using the latest waveform generation for PFA [13]. Non-PV-triggers may depend on left ventricular function [22], LA enlargement, degree of fibrosis or sex [23]. Freedom from AF, AFl or AT in patients with paroxysmal AF showed comparable rates in this trial (90 ± 6%) as previously reported (85 ± 5%) [13]. To our knowledge, we report here for the first time 1-year follow-up data of patients with persistent AF (freedom of AF, AFl and AT of 60 ± 10%), which seems comparable to previous reports on thermal ablation [24].
Technical considerations
Isolation of the RIPV was difficult in some patients: all 7 procedures with PFA catheter dwell times > 45 min (Fig. 4) were associated with difficulties in PFA catheter positioning along the respective RIPVs. In general, the right PVs needed more frequently additional PFA applications than left PV (Table 2). In the experience of the teams in Hamburg and Bad Oeynhausen, targeting an anterior transseptal puncture seems to facilitate positioning of the PFA catheter basket configuration with equal spline spacing.
We investigated systematically the effect of atropine on post-application vagal reaction after PFA pulses. Atropine reduced the duration of asystole and heart block as reported in single cases [25].
Treatment of non-PV-dependent atrial arrhythmias needs further investigation with regard to feasibility, efficacy and safety. In a recent publication coronary spasm after PFA application at the mitral isthmus was reported [26]. To the best of our knowledge, we report for the first time the PFA application at the SVC. In contrast to previous reports in swine [27], we observed a sinus pause of several minutes, which might be a direct effect on the sinus node due to its anatomical proximity or an indirect effect due to a vagal reaction. Further assessment of this potential treatment option is warranted.
Limitations
The current study gained important insights into real-world performance data using the novel Farapulse PFA ablation system in a clinical multicenter investigation. However, the current study is an observational, prospective, non-randomized analysis in two high-volume centers and in a limited number of patients.
Conclusions
In a multi-center real world setting, PVI by PFA is acutely highly effective, associated with a beneficial safety profile and a low recurrence rate.
Abbreviations
- 3D:
-
Three-dimensional
- AF:
-
Atrial fibrillation
- CTI:
-
Cavo-tricuspid isthmus ablation
- LA:
-
Left atrium
- LCPV:
-
Left common pulmonary vein
- LSPV:
-
Left superior pulmonary vein
- LIPV:
-
Left inferior pulmonary vein
- PFA:
-
Pulsed-field ablation
- PVI:
-
Pulmonary vein isolation
- PV:
-
Pulmonary vein
- RIPV:
-
Right inferior pulmonary vein
- RSPV:
-
Right superior pulmonary vein
- SVC:
-
Superior vena cava
References
Ramirez FD, Reddy VY, Viswanathan R, Hocini M, Jaïs P (2020) Emerging technologies for pulmonary vein isolation. Circ Res 127(1):170–183. https://doi.org/10.1161/CIRCRESAHA.120.316402
Metzner A, Reissmann B, Rausch P et al (2014) One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 7(2):288–292. https://doi.org/10.1161/CIRCEP.114.001473
Andrade JG, Monir G, Pollak SJ et al (2014) Pulmonary vein isolation using “contact force” ablation: the effect on dormant conduction and long-term freedom from recurrent atrial fibrillation–a prospective study. Heart Rhythm 11(11):1919–1924. https://doi.org/10.1016/j.hrthm.2014.07.033
Kuck K-H, Brugada J, Fürnkranz A et al (2016) Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 374(23):2235–2245. https://doi.org/10.1056/NEJMoa1602014
Calkins H, Hindricks G, Cappato R et al (2017) 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 33(5):369–409. https://doi.org/10.1016/j.joa.2017.08.001
Davalos RV, Mir LM, Rubinsky B (2005) Tissue ablation with irreversible electroporation. Ann Biomed Eng 33(2):223. https://doi.org/10.1007/s10439-005-8981-8
Nikolski VP, Efimov IR (2005) Electroporation of the heart. Europace. https://doi.org/10.1016/j.eupc.2005.04.011
Kotnik T, Kramar P, Pucihar G, Miklavcic D, Tarek M (2012) Cell membrane electroporation- part 1: the phenomenon. IEEE Electr Insul Mag 28(5):14–23. https://doi.org/10.1109/MEI.2012.6268438
Koruth JS, Kuroki K, Kawamura I et al (2020) Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol 13(3):e008303. https://doi.org/10.1161/CIRCEP.119.008303
Reddy VY, Anic A, Koruth J et al (2020) Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardial. https://doi.org/10.1016/j.jacc.2020.07.007
Kawamura I, Neuzil P, Shivamurthy P et al (2021) How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser )? Europace. https://doi.org/10.1093/europace/euab150
Kawamura I, Neuzil P, Shrivamurthy P et al (2021) Does pulsed fi eld ablation regress over time ? A quantitative temporal analysis of pulmonary vein isolation. Heart Rhythm. https://doi.org/10.1016/j.hrthm.2021.02.020
Reddy VY, Dukkipati SR, Neuzil P et al (2021) Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of impulse, pefcat, and pefcat II. JACC Clin Electrophysiol. 7(5):614–627. https://doi.org/10.1016/j.jacep.2021.02.014
Sørensen SK, Johannessen A, Worck R, Hansen ML, Hansen J (2021) Radiofrequency versus cryoballoon catheter ablation for paroxysmal atrial fibrillation: durability of pulmonary vein isolation and effect on atrial fibrillation burden: the race-AF randomized controlled trial. Circ Arrhythm Electrophysiol 14(5):e009573. https://doi.org/10.1161/CIRCEP.120.009573
Verma A, Boersma L, Haines DE et al (2022) First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the pulsed AF pilot trial. Circ Arrhythm Electrophysiol 15(1):e010168. https://doi.org/10.1161/CIRCEP.121.010168
Vogler J, Willems S, Sultan A et al (2015) Pulmonary vein isolation versus defragmentation: the chase-AF clinical trial. J Am Coll Cardiol 66(24):2743–2752. https://doi.org/10.1016/j.jacc.2015.09.088
Fink T, Schlüter M, Heeger C-H et al (2017) Stand-alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long-standing persistent atrial fibrillation: the randomized alster-lost-AF trial. Circ Arrhythm Electrophysiol. https://doi.org/10.1161/CIRCEP.117.005114
Kirchhof P, Camm AJ, Goette A et al (2020) Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 383(14):1305–1316. https://doi.org/10.1056/NEJMoa2019422
Wazni OM, Dandamudi G, Sood N et al (2021) Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 384(4):316–324. https://doi.org/10.1056/NEJMoa2029554
Andrade JG, Wells GA, Deyell MW et al (2021) Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 384(4):305–315. https://doi.org/10.1056/NEJMoa2029980
Reddy VY, Neuzil P, Koruth JS et al (2019) Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardial. https://doi.org/10.1016/j.jacc.2019.04.021
Zhao Y, Di Biase L, Trivedi C et al (2016) Importance of non-pulmonary vein triggers ablation to achieve long-term freedom from paroxysmal atrial fibrillation in patients with low ejection fraction. Heart Rhythm 13(1):141–149. https://doi.org/10.1016/j.hrthm.2015.08.029
Lee S-H, Tai C-T, Hsieh M-H et al (2005) Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol 46(6):1054–1059. https://doi.org/10.1016/j.jacc.2005.06.016
Lemes C, Wissner E, Lin T et al (2016) One-year clinical outcome after pulmonary vein isolation in persistent atrial fibrillation using the second-generation 28 mm cryoballoon: a retrospective analysis. Europace 18(2):201–205. https://doi.org/10.1093/europace/euv092
Gunawardene MA, Schaeffer BN, Jularic M et al (2022) Pulsed field ablation combined with ultra-high-density mapping in patients undergoing catheter ablation for atrial fibrillation: practical and electrophysiological considerations. J Cardiovasc Electrophysiol. https://doi.org/10.1111/jce.15349
Gunawardene MA, Schaeffer BN, Jularic M et al (2021) Coronary spasm during pulsed field ablation of the mitral isthmus line. JACC Clin Electrophysiol. https://doi.org/10.1016/j.jacep.2021.08.016
Koruth JS, Kuroki K, Iwasawa J et al (2020) Endocardial ventricular pulsed field ablation : a proof-of-concept preclinical evaluation. Europace. https://doi.org/10.1093/europace/euz341
Funding
Open Access funding enabled and organized by Projekt DEAL. Metzner received speaker's honoraria and travel grants from Medtronic, Biosense Webster, EPD Solutions/Philips (KODEX-EPD) and a research grant from Farapulse. A. Rillig: received travel grants, speaker and consultant fees from Biosense Webster, Medtronic, Cardiofocus, Ablamap and EPD Solutions/Philips (KODEX-EPD). B. Reissmann received speaker's honoraria and travel grants from Medtronic. C. Sohns received modest lecture honoraria and congress sponsoring from Abbott, Biosense Webster and Medtronic. M.D. Lemoine received a research grant from Farapulse was supported by the Research Promotion Fund of the Faculty of Medicine (Hamburg, “Clinician Scientist Program”). P. Kirchhof received research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last 3 years. PK is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). P. Sommer received speaker's honoraria and travel grants from Abbott, Boston, Medtronic, Biosense Webster, EPD Solutions/Philips.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The other authors report no conflicts.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lemoine, M.D., Fink, T., Mencke, C. et al. Pulsed-field ablation-based pulmonary vein isolation: acute safety, efficacy and short-term follow-up in a multi-center real world scenario. Clin Res Cardiol 112, 795–806 (2023). https://doi.org/10.1007/s00392-022-02091-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02091-2