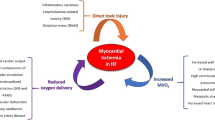
Abstract
Background
Patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) still experience a high rate of in-hospital complications. Liver fibrosis (LF) is a risk factor for mortality in the general population. We investigated whether the presence of LF detected by the validated fibrosis 4 (FIB-4) score may indicate ACS patients at higher risk of poor outcome.
Methods
In the prospective ongoing REAl-world observationaL rEgistry of Acute Coronary Syndrome (REALE-ACS), LF was defined by a FIB-4 score > 3.25. We repeated the analysis using an APRI score > 0.7. The primary endpoint was in-hospital adverse events (AEs) including a composite of in-hospital cardiogenic shock, PEA/asystole, acute pulmonary edema and death.
Results
A total of 469 consecutive ACS consecutive patients were enrolled. Overall, 21.1% of patients had a FIB-4 score > 3.25. Patients with LF were older, less frequently on P2Y12 inhibitors (p = 0.021) and admitted with higher serum levels of white blood cells (p < 0.001), neutrophils to lymphocytes ratio (p < 0.001), C-reactive protein (p = 0.013), hs-TnT (p < 0.001), creatine-kinase MB (p < 0.001), D-Dimer levels (p < 0.001). STEMI presentation and higher Killip class/GRACE score were more common in the LF group (p < 0.001). 71 patients experienced 110 AEs. At the multivariate analysis including clinical and laboratory risk factors, FIB-4 > 3.25 (OR 3.1, 95%CI 1.4–6.9), admission left ventricular ejection fraction% below median (OR 9.2, 95%CI 3.9–21.7) and Killip class ≥ II (OR 6.3, 95%CI 2.2–18.4) were the strongest independent predictors of in-hospital AEs. Similar results were obtained using the APRI score.
Conclusion
LF detected by FIB-4 score > 3.25 was associated with more severe ACS presentation and worse in-hospital AEs irrespective of clinical and laboratory variables.
Graphical abstract

Similar content being viewed by others
Introduction
Patients with acute coronary syndrome (ACS) still suffer from high in-hospital morbidity/mortality and long-term poor prognosis, including recurrence of myocardial infarction (MI) in about 10% of cases [1,2,3], despite advances in revascularization procedures techniques and current best medical therapy. This residual high cardiovascular risk may be driven by several factors including poor adherence to prescription [4], not reaching therapeutic target for comorbidities, or by the presence of non-cardiovascular risk factors [5], such as chronic kidney and liver disease.
In addition, a proportion of patients may experience early complications after ACS, including cardiogenic shock, arrhythmia, and in-hospital death. However, the clinical and biochemical characteristics of patients at risk of early complications after ACS are not well established, and so far, the use of common biomarkers of disease severity, such as high-sensitive troponin, did not lead to an improvement in clinical management of these patients [6].
In the past decades, given the impossibility of performing liver biopsies on large scale, different non-invasive biochemical markers have been proposed to identify patients with advanced liver damage. Among others, the AST-to-platelet ratio index (APRI) and the Fibrosis 4 (FIB-4) score, have been shown to be reliable tools for predicting liver fibrosis (LF) [7, 8].
Previous evidence also showed that a high FIB-4 was associated with an increased rate of long-term cardiovascular events in patients with cardiovascular disease, such as those affected by atrial fibrillation (AF) [9]. In addition, recent studies highlighted the association of LF with the severity of coronary artery disease (CAD) and advanced high-risk coronary plaque [10, 11].
Nevertheless, the impact of LF on in-hospital and short-term complications in patients admitted with ACS undergoing percutaneous coronary intervention (PCI) has been poorly investigated.
Our aim was to study the characteristics of patients hospitalized for ACS presenting with high FIB-4, and to investigate the relationship between LF and in-hospital outcomes.
Methods
The REAl-world observationaL rEgistry of Acute Coronary Syndrome (REALE-ACS) is an ongoing multicentric registry collecting data on characteristics, management and outcomes of consecutive patients admitted for ACS at the Department of Clinical Internal, Anesthesiologic, and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy and Emergency Medicine Unit Department (from January 2016).
Patients with age < 18 years were excluded. Diagnosis of non-ST-elevation ACS (i.e., unstable angina [UA] and non-ST-elevation myocardial infarction [NSTEMI]) and ST-elevation myocardial infarction (STEMI) were made according to the latest European Society of Cardiology guidelines [6, 12].
At baseline, demographic characteristics and clinical information of each study patient were recorded as following: age, sex, anthropometric data, cardiovascular risk factors (hypertension, diabetes mellitus, smoking (former + current), hypertriglyceridemia, hypercholesterolemia), previous history of CAD, heart failure, ischemic stroke, peripheral artery disease and Global Registry of Acute Coronary Events (GRACE) score. Standard 12-lead ECGs were collected at admission and during in-hospital stay.
Blood parameters
On admission, levels of aspartate aminotransferase AST/GOT (U/L), alanine aminotransferase ALT/GPT (U/L), C-reactive protein (CRP, upper limit of normal [ULN] < 0.5 mg/dL), hemoglobin (g/dL; anemia women < 12 g/dL and men < 13 g/dL), platelets × 109/L, white blood cells (WBCs) × 1000, neutrophils (%), lymphocytes (%), neutrophils/lymphocytes ratio, D-Dimer (ULN < 450 ng/mL), glycemia (mg/dL), creatinine (mg/dL), and high-sensitive troponin T (hs-TnT, ULN < 0.014 μg/L) were collected. Thrombocytopenia was defined by a platelet count < 150 × 109/L. Low serum albumin was defined as < 36 mg/L. Estimated glomerular filtration rates (eGFR) were determined using MDRD formula.
Definition of liver fibrosis
LF was defined by a FIB-4 score > 3.25, a validated non-invasive score determined by applying the following formula: (age [years] × AST [U/L])/(platelet count [109/L] × √ ALT [U/L]) [7, 8, 13]. We also used another non-invasive validated score to predict LF, namely APRI score by applying the following formula: (AST [U/L]/platelet count [109/L]) [7, 8, 13].
Clinical outcomes
The primary endpoint was a composite of in-hospital adverse events (AEs) including cardiogenic shock, pulseless electrical activity (PEA)/asystole, acute pulmonary edema and death. For the analysis, only the first event occurrence was considered.
The study was performed according to the Declaration of Helsinki.
Statistical analysis
Categorical variables were reported as counts and percentage. Continuous variables were expressed as mean and standard deviation and compared by Student t test. χ2 test was used to compare proportions. A first descriptive analysis according to presence of high FIB-4 was performed. We also described clinical and biochemical characteristics associated with in-hospital AEs. A multivariable logistic regression analysis was used to calculate the relative odds ratio (OR) and 95% confidence interval (95%CI) for each factor associated with in-hospital AEs.
In the multivariable model, only clinical variables with a p value < 0.100 at univariable analysis were included. In this model, we tested for collinearity to avoid an over adjustment of the model given the limited number of events. The variables entered in the multivariable model were FIB-4 > 3.25, below median of left ventricular ejection fraction (LVEF) value obtained for entry echocardiography, Killip Class ≥ 2, MDRD < 49 mL/min (value obtained from receiver operating characteristic [ROC] curve analysis to optimize the cut-off). We also performed a subgroup analysis on patients with albumin, CRP, and D-Dimer available values.
Only p values < 0.05 were considered as statistically significant. All tests were two tailed and analyses were performed using computer software packages (SPSS-25, SPSS Inc. and MedCalc).
Results
Clinical and laboratory findings
A total of 469 consecutive ACS patients were included. Among them, 12 patients had a history of chronic liver disease (6 patients viral hepatitis, 4 alcoholic disorder and 2 both). Clinical characteristics of patients with or without a high FIB-4 score are summarized in Table 1. Overall, 21.1% of patients had a high FIB-4 score. Patients with LF were older (p < 0.001), less frequently overweight (p = 0.026) and more frequently affected by permanent AF (p = 0.033).
Regarding pharmacological treatments at admission, patients with LF were less frequently on dual antiplatelet therapy (DAPT) (6% vs 13.9%, p = 0.060), especially P2Y12 inhibitor drugs (8.3% vs 19.3%, p = 0.021).
Patients with FIB-4 score > 3.25 had higher serum levels of WBCs (p < 0.001), neutrophils as % in WBC count (p < 0.001), CRP levels (p = 0.013), hs-TnT (p < 0.001), creatine-kinase MB (p < 0.001) and D-Dimer (p < 0.001). On the opposite, LF patients had lower percentage of lymphocytes (p < 0.001), platelets (p = 0.025), albumin levels (p = 0.018) on admission.
Supplementary Table 1 shows similar differences between patients with or without a high APRI score.
ACS presentation and angiographic features of patients with or without LF
Presentation of ACS was STEMI in 44.3%, NSTEMI in 29% and UA in 26.7% (Table 2). UA was more common in non-LF patients (33% vs 3%, p < 0.001), whilst STEMI was more common in the LF group (71.7% vs 37%, p < 0.001). Patients with LF had more severe ACS presentation, with a higher Killip class (34.4% vs 13.3%, p < 0.001) and GRACE score upon admission (161.82 ± 40.92 vs 126.12 ± 36.55, p = 0.001; GRACE > 140 71.7% vs 63.5%, p < 0.001). No differences between the two groups were reported when analyzing angiographic data (Table 2).
ACS and angiographic characteristics between patients with high or low APRI score were consistent with those obtained by using FIB-4 score (Supplementary Table 2).
In-hospital adverse events
Overall, 71 patients experienced 110 AEs. The number and type of AEs according to presence or absence of a high FIB-4 score are reported in Table 3.
Table 4 reports clinical events occurring during the in-hospital staying according to the presence or absence of LF. Noteworthy, AEs were significantly more frequent in patients with LF than in those without (26.3% vs 12.2%, respectively, p = 0.001), with this difference being more evident in STEMI patients (Supplementary Fig. 1). In particular, cardiogenic shock (17.2% vs 7.3%, p = 0.006) and PEA/asystole (8.1% vs 3.0%, p = 0.039) were more frequent in LF patients, while cardiac deaths and acute pulmonary edema did not differ between the two groups.
Table 4 shows characteristics of patients experiencing or not AEs during hospitalization. Patients suffering from in-hospital AEs were characterized by an older age, longer in-hospital stay, lower values of LVEF, higher GRACE score and had more frequently STEMI and cardiogenic shock presentation. At laboratory analysis on admission, they also showed more frequently hypoalbuminemia and higher serum values of CRP, AST, WBC count, neutrophils/lymphocytes ratio, D-Dimer, glucose, hs-TnT and CK-MB (Table 4).
Table 5 shows univariable and multivariable odds ratio (OR) and 95% confidence interval (95% CI) of factors associated with in-hospital AEs.
At the multivariable analysis, FIB-4 was found to be and independent predictor of poor outcomes (OR 3.1, 95%CI 1.4–6.9). Admission LVEF% below median was the more relevant independent predictor of in-hospital AEs (OR 9.2, 95%CI 3.9–21.7) followed by a Killip class ≥ II (OR 6.3, 95%CI 2.2–18.4) (Table 5). Moreover, eGFR MDRD < 49 ml/min (OR 2.4, 95%CI 1.02–5.5), albumin below median (OR 2.2, 95%CI 1.03–4.7) and CRP above median (OR 2.2, 95%CI 1.02–5) were also associated with in-hospital outcomes (Table 5).
When we repeated the analysis using the negative value of FIB-4 < 1.45 (rule out criterion for the presence of LF), we found an inverse association between low FIB-4 score and AEs at univariable OR: 0.45, 95%CI 0.24–0.86, p = 0.015, a marginally significant value at multivariable analysis (using Model A variables) with OR 0.55, 95%CI 0.30–1.01, p = 0.055).
Discussion
The main findings of our prospective cohort study were that non-invasive diagnosis of LF was achieved in one out of five ACS patients, identifying patients with severe MI and at higher risk of in-hospital adverse events independent of traditional clinical and laboratory risk factors.
In our study on 469 patients with ACS, we found a 21.1% of LF prevalence as defined by a FIB-4 score > 3.25. In a study including 3263 patients with CAD in China, 1035 (31.7%) had a high FIB-4 defined by a value > 2.67 [14].
We found that patients with LF had a more severe ACS presentation. First, they suffered more frequently from hypoalbuminemia, thrombocytopenia and highest levels of inflammatory/severity markers such as hs-TnT, CRP, neutrophils to lymphocytes ratio and D-Dimer, all biomarkers associated with poor prognosis in ACS setting [15,16,17,18,19]. Then, in our population, MI clinical presentation was worse in LF patients. Indeed, they were more frequently admitted with STEMI and a higher Killip class and GRACE score. This is consistent with previous studies analyzing only STEMI patients who showed a very high percentage of LF/liver steatosis patients among them [11, 20].
We found a similar proportion of multivessel disease between patients with and without high FIB-4, that may be probably due to the high percentage of STEMI in the former group.
Interestingly, LF patients were less frequently on DAPT therapy, especially P2Y12 inhibitors, on admission. The association between antiplatelet therapy and LF has been investigated in the past and a protective association between the use of antiplatelet agents and the occurrence of liver fibrosis has been demonstrated in a prospective cohort study of patients at high risk of cardiovascular events [21]. Previous data have shown a pathogenic role of platelets in the pathogenesis of LF, and antiplatelet agents was found to be effective in attenuating liver steatosis in preclinical studies [22, 23].
We also investigated the association between LF and in-hospital outcomes. We found that LF as detected by an easy inexpensive non-invasive score calculated with routine laboratory data was independently associated with in-hospital AEs independently from traditional clinical and laboratory risk factors. The association between LF and mortality has been investigated only in one previous study performed in China using a lower FIB-4 cut-off, in which Chen et al. found that higher LF scores were associated with increased risks of all-cause and cardiovascular mortality among CAD patients followed for 7.5 years [14].
The pathophysiology linking between LF and CAD is unclear but may rely on different mechanisms. LF and eventually liver steatosis have been associated with increased levels of pro-inflammatory cytokines, such as tumor necrosis factor α, interleukin 6, and CRP, biomarkers well known to have an impact on CAD and cardiovascular prognosis [24]. As well, advanced LF has been shown to be associated with endothelial dysfunction increasing atherosclerosis [25]. Previous studies have shown an association between LF assessed with non-invasive markers and imaging indicators of atherosclerosis such as carotid mean intimal thickness and coronary calcium score [26, 27].
Novel pathogenetic insights come from the study of gut and gut-derived products. Thus, an increased liver localization of gut-derived lipopolysaccharides (LPS) has been found in patients with liver disease, associated with increased liver inflammation [28].
Additionally, a growing body of evidence suggest that LPS may contribute to increase cardiovascular risk favoring platelet activation [29, 30] and that LPS may found into human atherosclerotic plaque potentially contributing to plaque vulnerability and in turn favoring plaque rupture [31, 32]. Indeed, in ACS patients with LF, LPS may contribute to develop a more severe coronary disease. This aspect deserves further study.
Our study had some limitations. First, this was a single-center study and results must be confirmed in larger studies. Although liver biopsy is the best standard to confirm/exclude LF, it is invasive, expensive, prone to sampling error, and can cause rare but potentially life-threatening complications. Nowadays, it has been almost entirely replaced by non-invasive methods that measure liver stiffness, such as transient elastography, or biochemical markers and scoring systems. In terms of cost-effectiveness, considering the high prevalence of LF and CAD, biochemical markers, such as the APRI score and the FIB-4 score, recently shown to be reliable in predicting LF and validated in NAFLD population [13, 33], may be helpful to identify ACS patients at higher risk of complications and events. Furthermore, in our analysis a limited number of patients had a known liver disease (viral or/and alcoholic) that does not allow a specific analysis on the subtypes of LF. Further studies are needed to investigate the impact of different liver conditions on ACS patients.
In conclusion, LF was associated with a more severe presentation and worse in-hospital AEs in ACS patients undergoing PCI. Our findings suggest that the detection of LF with non-invasive and inexpensive scores may represent a simple method to improve risk stratification and management of patients with ACS.
Abbreviations
- ACS:
-
Acute coronary syndrome
- AEs:
-
Adverse events
- AF:
-
Atrial fibrillation
- ALT:
-
Alanine aminotransferase
- APRI:
-
AST-to-platelet ratio index
- AST:
-
Aspartate aminotransferase
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- DAPT:
-
Dual antiplatelet therapy
- eGFR:
-
Estimated glomerular filtration rates
- FIB-4:
-
Fibrosis 4
- GRACE:
-
Global Registry of Acute Coronary Events
- hs-TnT:
-
High-sensitive troponin T
- LVEF:
-
Left ventricular ejection fraction
- LF:
-
Liver fibrosis
- MI:
-
Myocardial infarction
- NSTEMI:
-
Non-ST-elevation myocardial infarction
- OR:
-
Odds ratio
- PCI:
-
Percutaneous coronary intervention
- PEA:
-
Pulseless electrical activity
- ROC:
-
Receiver operating characteristic
- STEMI:
-
ST-elevation myocardial infarction
- UA:
-
Unstable angina
- WBCs:
-
White blood cells
References
Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M (2016) Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 37(42):3232–3245. https://doi.org/10.1093/eurheartj/ehw334
Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M (2015) Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 36(19):1163–1170. https://doi.org/10.1093/eurheartj/ehu505
Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ (2015) Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 132(17):1667–1678. https://doi.org/10.1161/circulationaha.114.008720
Hirsh BJ, Smilowitz NR, Rosenson RS, Fuster V, Sperling LS (2015) Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol 66(2):184–192. https://doi.org/10.1016/j.jacc.2015.05.030
Canivell S, Muller O, Gencer B, Heg D, Klingenberg R, Räber L, Carballo D, Matter C, Lüscher T, Windecker S, Mach F, Rodondi N, Nanchen D (2018) Prognosis of cardiovascular and non-cardiovascular multimorbidity after acute coronary syndrome. PLoS ONE 13(4):e0195174. https://doi.org/10.1371/journal.pone.0195174
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM (2020) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa575
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55(6):2005–2023. https://doi.org/10.1002/hep.25762
Papadopoulos N, Vasileiadi S, Papavdi M, Sveroni E, Antonakaki P, Dellaporta E, Koutli E, Michalea S, Manolakopoulos S, Koskinas J, Deutsch M (2019) Liver fibrosis staging with combination of APRI and FIB-4 scoring systems in chronic hepatitis C as an alternative to transient elastography. Ann Gastroenterol 32(5):498–503. https://doi.org/10.20524/aog.2019.0406
Pastori D, Lip GYH, Farcomeni A, Del Sole F, Sciacqua A, Perticone F, Marcucci R, Grifoni E, Pignatelli P, Violi F, group A-As (2018) Incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin K or non-vitamin K antagonist oral anticoagulants. Int J Cardiol 264:58–63. https://doi.org/10.1016/j.ijcard.2018.01.097
Turan Y (2020) The nonalcoholic fatty liver disease fibrosis score is related to epicardial fat thickness and complexity of coronary artery disease. Angiology 71(1):77–82. https://doi.org/10.1177/0003319719844933
Ismaiel A, Popa SL, Dumitrascu DL (2020) Acute coronary syndromes and nonalcoholic fatty liver disease: “un affaire de coeur.” Can J Gastroenterol Hepatol 2020:8825615. https://doi.org/10.1155/2020/8825615
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177. https://doi.org/10.1093/eurheartj/ehx393
Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ (2009) Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 7(10):1104–1112. https://doi.org/10.1016/j.cgh.2009.05.033
Chen Q, Li Q, Li D, Chen X, Liu Z, Hu G, Wang J, Ling W (2020) Association between liver fibrosis scores and the risk of mortality among patients with coronary artery disease. Atherosclerosis 299:45–52. https://doi.org/10.1016/j.atherosclerosis.2020.03.010
Bicciré FG, Pastori D, Tanzilli A, Pignatelli P, Viceconte N, Barillà F, Versaci F, Gaudio C, Violi F, Tanzilli G (2021) Low serum albumin levels and in-hospital outcomes in patients with ST segment elevation myocardial infarction. Nutr Metab Cardiovasc Dis 31(10):2904–2911. https://doi.org/10.1016/j.numecd.2021.06.003
Zhang X, Wang S, Fang S, Yu B (2021) Prognostic role of high sensitivity C-reactive protein in patients with acute myocardial infarction. Front Cardiovasc Med 8:659446–659446. https://doi.org/10.3389/fcvm.2021.659446
Zhang S, Diao J, Qi C, Jin J, Li L, Gao X, Gong L, Wu W (2018) Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord 18(1):75. https://doi.org/10.1186/s12872-018-0812-6
Biccirè FG, Farcomeni A, Gaudio C, Pignatelli P, Tanzilli G, Pastori D (2021) D-dimer for risk stratification and antithrombotic treatment management in acute coronary syndrome patients: asystematic review and metanalysis. Thromb J 19(1):102. https://doi.org/10.1186/s12959-021-00354-y
Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS (2012) How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 33(18):2252–2257. https://doi.org/10.1093/eurheartj/ehs154
Boddi M, Tarquini R, Chiostri M, Marra F, Valente S, Giglioli C, Gensini GF, Abbate R (2013) Nonalcoholic fatty liver in nondiabetic patients with acute coronary syndromes. Eur J Clin Invest 43(5):429–438. https://doi.org/10.1111/eci.12065
Schwarzkopf K, Bojunga J, Rüschenbaum S, Martinez Y, Mücke MM, Seeger F, Schoelzel F, Zeuzem S, Friedrich-Rust M, Lange CM (2018) Use of antiplatelet agents is inversely associated with liver fibrosis in patients with cardiovascular disease. Hepatol Commun 2(12):1601–1609. https://doi.org/10.1002/hep4.1254
Chauhan A, Adams DH, Watson SP, Lalor PF (2016) Platelets: No longer bystanders in liver disease. Hepatology 64(5):1774–1784. https://doi.org/10.1002/hep.28526
Fujita K, Nozaki Y, Wada K, Yoneda M, Endo H, Takahashi H, Iwasaki T, Inamori M, Abe Y, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nagashima Y, Nakajima A (2008) Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut 57(11):1583–1591. https://doi.org/10.1136/gut.2007.144550
Jichitu A, Bungau S, Stanescu AMA, Vesa CM, Toma MM, Bustea C, Iurciuc S, Rus M, Bacalbasa N, Diaconu CC (2021) Non-alcoholic fatty liver disease and cardiovascular comorbidities: pathophysiological links, diagnosis, and therapeutic management. Diagnostics. https://doi.org/10.3390/diagnostics11040689
Barone M, Viggiani MT, Amoruso A, Schiraldi S, Zito A, Devito F, Cortese F, Gesualdo M, Brunetti N, Di Leo A, Scicchitano P, Ciccone MM (2015) Endothelial dysfunction correlates with liver fibrosis in chronic HCV infection. Gastroenterol Res Pract 2015:682174–682174. https://doi.org/10.1155/2015/682174
Song DS, Chang UI, Kang SG, Song SW, Yang JM (2019) Noninvasive serum fibrosis markers are associated with coronary artery calcification in patients with nonalcoholic fatty liver disease. Gut Liver 13(6):658–668. https://doi.org/10.5009/gnl18439
Sesti G, Sciacqua A, Fiorentino TV, Perticone M, Succurro E, Perticone F (2014) Association between noninvasive fibrosis markers and cardio-vascular organ damage among adults with hepatic steatosis. PLoS ONE 9(8):e104941–e104941. https://doi.org/10.1371/journal.pone.0104941
Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, Francis H, Cardinale V, Onori P, Safarikia S, Cammisotto V, Alvaro D, Svegliati-Baroni G, Angelico F, Gaudio E, Violi F (2020) Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology 72(2):470–485. https://doi.org/10.1002/hep.31056
Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, Menichelli D, Pignatelli P, Violi F (2017) Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation effect of adherence to mediterranean diet. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.005784
Nocella C, Carnevale R, Bartimoccia S, Novo M, Cangemi R, Pastori D, Calvieri C, Pignatelli P, Violi F (2017) Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb Haemost 117(8):1558–1570. https://doi.org/10.1160/TH16-11-0857
Ni M, Wang Y, Zhang M, Zhang PF, Ding SF, Liu CX, Liu XL, Zhao YX, Zhang Y (2009) Atherosclerotic plaque disruption induced by stress and lipopolysaccharide in apolipoprotein E knockout mice. Am J Physiol Heart Circ Physiol 296(5):H1598-1606. https://doi.org/10.1152/ajpheart.01202.2008
Carnevale R, Nocella C, Petrozza V, Cammisotto V, Pacini L, Sorrentino V, Martinelli O, Irace L, Sciarretta S, Frati G, Pastori D, Violi F (2018) Localization of lipopolysaccharide from Escherichia Coli into human atherosclerotic plaque. Sci Rep 8(1):3598. https://doi.org/10.1038/s41598-018-22076-4
McPherson S, Stewart SF, Henderson E, Burt AD, Day CP (2010) Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 59(9):1265–1269. https://doi.org/10.1136/gut.2010.216077
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. The study was supported by the Grant Bando di Ateneo Medio 2022 assigned to Prof. Daniele Pastori (protocol number RM12117A82C9EE21).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biccirè, F.G., Barillà, F., Sammartini, E. et al. Relationship between non-invasively detected liver fibrosis and in-hospital outcomes in patients with acute coronary syndrome undergoing PCI. Clin Res Cardiol 112, 236–246 (2023). https://doi.org/10.1007/s00392-022-02078-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02078-z