Abstract
Background
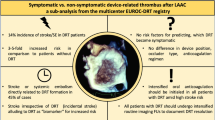
Data on Device-related Thrombus (DRT) after left atrial appendage closure (LAAC) remain scarce. This study aimed to investigate risk factors for DRT from centers reporting to the EUROC-DRT registry.
Methods
We included 537 patients (112 with DRT and 425 without DRT) who had undergone LAAC between 12/2008 and 04/2019. Baseline and implantation characteristics, anti-thrombotic treatment and clinical outcomes were compared between both groups in uni- and multivariate analyses. Additional propensity-score matching (PSM) was conducted to focus on the role of implantation characteristics.
Results
Patients with DRT showed higher rates of previous stroke/transient ischemic attack (TIA) (49.1% vs. 34.7%, p < 0.01), spontaneous echocardiographic contrast (SEC) (44.9% vs. 27.7%, p < 0.01) and lower left atrial appendage (LAA) peak emptying velocity (35.4 ± 18.5 vs. 42.4 ± 18.0 cm/s, p = 0.02). Occluders implanted in DRT patients were larger (25.5 ± 3.8 vs. 24.6 ± 3.5 mm, p = 0.03) and implanted deeper in the LAA (mean depth: 7.6 ± 4.7 vs. 5.7 ± 4.7 mm, p < 0.01). Coverage of the appendage ostium was achieved less often in DRT patients (69.5% vs. 81.5%, p < 0.01), while DRT patients were less frequently on oral anticoagulation (7.1% vs. 16.7%, p < 0.01). Multivariate analysis identified age, prior stroke/TIA and SEC as independent risk factors for DRT. After PSM, implantation depth was found to be predictive. Rates of stroke/TIA were higher in DRT patients (13.5% vs. 3.8%, Hazard Ratio: 4.21 [95%-confidence interval: 1.88–9.49], p < 0.01).
Conclusions
DRT after LAAC is associated with adverse outcome and appears to be of multifactorial origin, depending on patient characteristics, anticoagulation regimen and device position.
Graphical abstract



Similar content being viewed by others
Abbreviations
- AF:
-
Atrial fibrillation
- CI:
-
Confidence interval
- CT:
-
Computer tomography
- DAPT:
-
Dual antiplatelet therapy
- DRT:
-
Device-related thrombosis
- FU:
-
Follow-up
- LA:
-
Left atrium
- LAA:
-
Left atrial appendage
- LAAC:
-
Left atrial appendage closure
- LUPV:
-
Left upper pulmonary vein
- NOAC:
-
Novel oral anticoagulation
- OAC:
-
Oral anticoagulation
- OR:
-
Odds ratio
- PSM:
-
Propensity score matching
- SAPT:
-
Single antiplatelet therapy
- SEC:
-
Spontaneous echocardiographic contrast
- TEE:
-
Transoesophageal echocardiography
- TIA:
-
Transient ischaemic attack
- VKA:
-
Vitamin K antagonist
References
Hildick-Smith D, Landmesser U, Camm AJ et al (2020) Left atrial appendage occlusion with the Amplatzer™ Amulet™ device: full results of the prospective global observational study. Eur Heart J 41:2894–2901. https://doi.org/10.1093/eurheartj/ehaa169
Holmes DR, Reddy VY, Turi ZG et al (2009) Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374:534–542. https://doi.org/10.1016/S0140-6736(09)61343-X
Cochet H, Iriart X, Sridi S et al (2018) Left atrial appendage patency and device-related thrombus after percutaneous left atrial appendage occlusion: a computed tomography study. Eur Heart J Cardiovasc Imaging 19:1351–1361. https://doi.org/10.1093/ehjci/jey010
Fauchier L, Cinaud A, Brigadeau F et al (2018) Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol 71:1528–1536. https://doi.org/10.1016/j.jacc.2018.01.076
Lempereur M, Aminian A, Saw J (2018) Device-associated thrombus formation after left atrial appendage occlusion: a systematic review of events reported with the Watchman, the Amplatzer Cardiac Plug and the Amulet. Catheter Cardiovasc Interv 92:E216–E217. https://doi.org/10.1002/ccd.27135
Sedaghat A, Schrickel J, Andrié R et al (2016) Thrombus formation after left atrial appendage occlusion with the amplatzer amulet device. JACC Clin Electrophysiol. https://doi.org/10.1016/j.jacep.2016.05.006
Dukkipati SR, Kar S, Holmes DR et al (2018) Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation 138:874–885. https://doi.org/10.1161/CIRCULATIONAHA.118.035090
Sedaghat A, Nickenig G, Schrickel JW et al (2021) Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device-Insights from the EWOLUTION real world registry. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. https://doi.org/10.1002/ccd.29458
Freixa X, Cepas-Guillen P, Flores-Umanzor E et al (2021) Pulmonary ridge coverage and device-related thrombosis after left atrial appendage occlusion. EuroIntervention 16:E1288–E1294. https://doi.org/10.4244/EIJ-D-20-00886
Sedaghat A, Vij V, Al-Kassou B et al (2021) Device-related thrombus after left atrial appendage closure: data on thrombus characteristics, treatment strategies, and clinical outcomes from the EUROC-DRT-Registry. Circ Cardiovasc Interv. https://doi.org/10.1161/circinterventions.120.010195
Aminian A, Schmidt B, Mazzone P et al (2019) Incidence, characterization, and clinical impact of device-related thrombus following left atrial appendage occlusion in the prospective global AMPLATZER amulet observational study. JACC Cardiovasc Interv 12:1003–1014. https://doi.org/10.1016/j.jcin.2019.02.003
Pracon R, Bangalore S, Dzielinska Z et al (2018) Device thrombosis after percutaneous left atrial appendage occlusion is related to patient and procedural characteristics but not to duration of postimplantation dual antiplatelet therapy. Circ Cardiovasc Interv 11:1–7. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005997
Simard T, Jung RG, Lehenbauer K et al (2021) Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol 78:297–313. https://doi.org/10.1016/j.jacc.2021.04.098
Plicht B, Konorza TFM, Kahlert P et al (2013) Risk factors for thrombus formation on the amplatzer cardiac plug after left atrial appendage occlusion. JACC Cardiovasc Interv 6:606–613. https://doi.org/10.1016/j.jcin.2013.02.014
Rashid HN, Layland J (2021) Association between device-related thrombus and the neo-appendage with left-atrial appendage occlusion devices. Eur Heart J 42:1047–1048. https://doi.org/10.1093/eurheartj/ehaa803
Mill J, Olivares AL, Arzamendi D et al (2020) Impact of flow dynamics on device-related thrombosis after left atrial appendage occlusion. Can J Cardiol 36:968.e13-968.e14. https://doi.org/10.1016/j.cjca.2019.12.036
Meier B (2018) What lies beneath left atrial appendage occlusion. Circulation 11:1–3. https://doi.org/10.1161/CIRCINTERVENTIONS.118.006360
Søndergaard L, Wong YH, Reddy VY et al (2019) Propensity-matched comparison of oral anticoagulation versus antiplatelet therapy after left atrial appendage closure with WATCHMAN. JACC Cardiovasc Interv 12:1055–1063. https://doi.org/10.1016/j.jcin.2019.04.004
Duthoit G, Silvain J, Marijon E et al (2020) Reduced rivaroxaban dose versus dual antiplatelet therapy after left atrial appendage closure: ADRIFT a randomized pilot study. Circ Cardiovasc Interv 13:e008481. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008481
Garot P, Iriart X, Aminian A et al (2020) Value of FEops HEARTguide patient-specific computational simulations in the planning of left atrial appendage closure with the Amplatzer Amulet closure device: rationale and design of the PREDICT-LAA study. Open Hear 7:1–5. https://doi.org/10.1136/openhrt-2020-001326
Acknowledgements
We thank Dr. Marie-Therese Schmitz and the Institute for Biometry of the University Bonn (IMBIE) for statistical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alexander Sedaghat has received travel grants from Abbott and Boston Scientific and is a proctor for Lifetech. Lars Sondergaard has received consultant fees and institutional research grants from Abbott and Boston Scientific, and is shareholder in Eclipse Medical. Dr Cruz-Gonzalez is a proctor for Abbott, Boston Scientific and Lifetech and was funded by ISCIII (PI19/00658) and co-funded by ERDF, "A way to make Europe". Jens Erik Nielsen-Kudsk is a proctor and consultant for Abbott and Boston Scientific. Dabit Arzamendi is a proctor for Abbott and Boston Scientific. Xavier Freixa is a proctor for Abbott, Boston Scientific and Lifetech. Antonio Mangieri is part of the advisory board of Boston Scientific and received an institutional grant from Boston Scientific. Dr. Nombela-Franco has served as a proctor of Abbott Vascular and received speaker honoraria from Boston Scientific and Abbott Vascular. Dr. Meier has received consultant fees from Abbott. Xavier Iriart is a proctor for Abbott and Boston Scientific. Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent.) Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vij, V., Piayda, K., Nelles, D. et al. Clinical and echocardiographic risk factors for device-related thrombus after left atrial appendage closure: an analysis from the multicenter EUROC-DRT registry. Clin Res Cardiol 111, 1276–1285 (2022). https://doi.org/10.1007/s00392-022-02065-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02065-4