Abstract
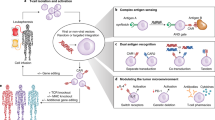
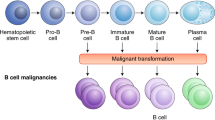
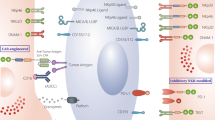
This year marks the tenth anniversary of cell therapy with chimeric antigen receptor (CAR)-modified T cells for refractory leukemia. The widespread commercial approval of genetically engineered T cells for a variety of blood cancers offers hope for patients with other types of cancer, and the convergence of human genome engineering and cell therapy technology holds great potential for generation of a new class of cellular therapeutics. In this Review, we discuss the goals of cellular immunotherapy in cancer, key challenges facing the field and exciting strategies that are emerging to overcome these obstacles. Finally, we outline how developments in the cancer field are paving the way for cellular immunotherapeutics in other diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Combination Products (FDA, accessed 1 March 2022); https://www.fda.gov/combination-products
Grupp,S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Fischbach, M. A., Bluestone, J. A. & Lim, W. A. Cell-based therapeutics: the next pillar of medicine. Sci. Transl. Med 5, 179ps177 (2013).
Frangoul, H. et al. CRISPR–Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 384, 252–260 (2021).
Approved Cellular and Gene Therapy (FDA, 2022); https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
Elisseeff, J., Badylak, S. F. & Boeke, J. D. Immune and genome engineering as the future of transplantable tissue. N. Engl. J. Med. 385, 2451–2462 (2021).
Benjamin, R. et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894 (2020).
Anderson, D., Billingham, R. E., Lampkin, G. H. & Medawar, P. B. The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity 5, 379–397 (1951).
Martínez‐Llordella, M. et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am. J. Transplant. 7, 309–319 (2007).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer–immunity cycle. Immunity 39, 1–10 (2013).
Beatty, G. L. & Gladney, W. L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 21, 687–692 (2015).
Cudkowicz, G. & Stimpfling, J. Deficient growth of C57bl marrow cells transplanted in F1 hybrid mice: association with the histocompatibility-2 locus. Immunology 7, 291 (1964).
Murphy, W. J., Kumar, V. & Bennett, M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. J. Exp. Med. 166, 1499–1509 (1987).
Kiessling, R. et al. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur. J. Immunol. 7, 655–663 (1977).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002).
Huntington, N. D., Vosshenrich, C. A. & Di Santo, J. P. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7, 703–714 (2007).
Raulet, D. H. Bone marrow cell rejection, MHC, NK cells, and missing self recognition: ain’t that peculiar (with apologies to Marvin Gaye). J. Immunol. 195, 2923–2925 (2015).
Liu, W., Xiao, X., Demirci, G., Madsen, J. & Li, X. C. Innate NK cells and macrophages recognize and reject allogeneic nonself in vivo via different mechanisms. J. Immunol. 188, 2703–2711 (2012).
Marino, J., Paster, J. & Benichou, G. Allorecognition by T lymphocytes and allograft rejection. Front. Immunol. 7, 582 (2016).
Kanda, Y. et al. Visualizing the rapid and dynamic elimination of allogeneic T cells in secondary lymphoid organs. J. Immunol. 201, 1062–1072 (2018).
Kitazawa, Y. et al. Novel targeting to XCR1+ dendritic cells using allogeneic T cells for polytopical antibody responses in the lymph nodes. Front. Immunol. 10, 1195 (2019).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Ringdén, O., Karlsson, H., Olsson, R., Omazic, B. & Uhlin, M. The allogeneic graft‐versus‐cancer effect. Br. J. Haematol. 147, 614–633 (2009).
Chapuis, A. G. et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med 25, 1064–1072 (2019).
Wei, Q. & Frenette, P. S. Niches for hematopoietic stem cells and their progeny. Immunity 48, 632–648 (2018).
Yu, V. W. & Scadden, D. T. Heterogeneity of the bone marrow niche. Curr. Opin. Hematol. 23, 331 (2016).
Khaldoyanidi, S., Nagorsen, D., Stein, A., Ossenkoppele, G. & Subklewe, M. Immune biology of acute myeloid leukemia: implications for immunotherapy. J. Clin. Oncol. 39, 419–432 (2021).
Passegué, E., Jamieson, C. H., Ailles, L. E. & Weissman, I. L. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl Acad. Sci. USA 100, 11842–11849 (2003).
Paszkiewicz, P. J. et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Invest. 126, 4262–4272 (2016).
Melenhorst, J. J. et al. Decade-long remissions of leukemia sustained by the persistence of activated CD4+ CAR T-cells. Nature 602, 503–509 (2022).
Ma, L. et al. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019).
Reinhard, K. et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020).
Hanahan, D. & Coussens L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Jain, R. K., Martin, J. D. & Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014).
Seitter, S. J. et al. Impact of prior treatment on the efficacy of adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma. Clin. Cancer Res. 27, 5289–5298 (2021).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–529 (2015).
Brown, L. V., Gaffney, E. A., Ager, A., Wagg, J. & Coles, M. C. Quantifying the limits of CAR T-cell delivery in mice and men. J. R. Soc. Interface 18, 20201013 (2021).
Majzner, R. G. & Mackall, C. L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25, 1341–1355 (2019).
Thommen, D. S. & Schumacher, T. N. T cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018).
Pauken, K. E. & Wherry, E. J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36, 265–276 (2015).
Hong, M., Clubb, J. D. & Chen, Y. Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 38, 473–488 (2020).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Good, C. R. et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell 184, 6081–6100 (2021).
June, C. H., Warshauer, J. T. & Bluestone, J. A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med 23, 540–547 (2017).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 25, 625–638 (2019).
Sheth, V. S. & Gauthier, J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 56, 552–566 (2021).
Taraseviciute, A. et al. Chimeric antigen receptor t cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 8, 750–763 (2018).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e117 (2020).
Lareau, C. A., Parker, K. R. & Satpathy, A. T. Charting the tumor antigen maps drawn by single-cell genomics. Cancer Cell 39, 1553–1557 (2021).
Van Oekelen, O. et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med. 27, 2099–2103 (2021).
Cameron, B. J. et al. Identification of a titin-derived HLA-A1–presented peptide as a cross-reactive target for engineered MAGE A3–directed T cells. Sci. Transl. Med. 5, 197ra103 (2013).
Tang, K. & Nastoupil, L. J. Real-world experiences of CAR T-cell therapy for large B-cell lymphoma: how similar are they to the prospective studies? J. Immunother. Precis. Oncol. 4, 150–159 (2021).
Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 22, 216–234 (2021).
Scholler, J. et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 4, 132Ra153 (2012).
Micklethwaite, K. P. et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood 138, 1391–1405 (2021).
Schambach, A., Morgan, M. & Fehse, B. Two cases of T cell lymphoma following Piggybac-mediated CAR T cell therapy. Mol. Ther. 292, 631–633 (2021).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Nori, S. et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 4, 360–373 (2015).
Ben-David, U. & Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 11, 268–277 (2011).
Hataye, J., Moon, J. J., Khoruts, A., Reilly, C. & Jenkins, M. K. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312, 114–116 (2006).
Newrzela, S. et al. Resistance of mature T cells to oncogene transformation. Blood 112, 2278–2286 (2008).
Kaldor, J. M. et al. Leukemia following chemotherapy for ovarian cancer. N. Engl. J. Med 322, 1–6 (1990).
Ajina, A. & Maher, J. Prospects for combined use of oncolytic viruses and CAR T-cells. J. Immunother. Cancer 5, 90 (2017).
Guedan, S. & Alemany, R. CAR-T cells and oncolytic viruses: joining forces to overcome the solid tumor challenge. Front Immunol. 9, 2460 (2018).
Biegert, G. W. G., Shaw, A. R. & Suzuki, M. Current development in adenoviral vectors for cancer immunotherapy. Mol. Ther. Oncolytics 23, 571–581 (2021).
Adusumilli, P. S. et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 11, 2748–2763 (2021).
Choi, B. D. et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019).
Siurala, M. et al. Adenoviral delivery of tumor necrosis factor-alpha and interleukin-2 enables successful adoptive cell therapy of immunosuppressive melanoma. Mol. Ther. 24, 1435–1443 (2016).
Rosewell Shaw, A. et al. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T Cells against metastatic head and neck cancer. Mol. Ther. 25, 2440–2451 (2017).
Kim, K. H. et al. A phase I clinical trial of Ad5/3-∆24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol. 130, 518–524 (2013).
Ranki, T. et al. Phase I study with ONCOS-102 for the treatment of solid tumors — an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 4, 17 (2016).
Park, A.K., et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 12, eaaz1863 (2020).
Watanabe, K. et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 3, e99573 (2018).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–856 (2018).
Xia, Y., Medeiros, L. J. & Young, K. H. Immune checkpoint blockade: releasing the brake towards hematological malignancies. Blood Rev. 30, 189–200 (2016).
Yin, Y. et al. Checkpoint blockade reverses anergy in IL-13Rα2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol. Ther. Oncolytics 11, 20–38 (2018).
Wing, A. et al. Improving CAR T-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol. Res. 6, 605–616 (2018).
Friedmann, T. & Roblin, R. Gene therapy for human genetic disease? Science 175, 949–955 (1972).
Williams, T. N. & Weatherall, D. J. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb. Perspect. Med. 2, a011692 (2012).
Ndung’u, T., McCune, J. M. & Deeks, S. G. Why and where an HIV cure is needed and how it might be achieved. Nature 576, 397–405 (2019).
Pellenz, S. et al. New human chromosomal sites with “safe harbor” potential for targeted transgene insertion. Hum. Gene Ther. 30, 814–828 (2019).
Kim, M. Y. et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell 173, 1439–1453.e1419 (2018).
Dobzhansky, T. Genetics of natural populations. XIII. Recombination and variability in populations of Drosophila pseudoobscura. Genetics 31, 269 (1946).
Setton, J. et al. Synthetic lethality in cancer therapeutics: the next generation. Cancer Discov. 11, 1626–1635 (2021).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Klebanoff, C. A. & Wolchok, J. D. Shared cancer neoantigens: making private matters public. J. Exp. Med. 215, 5–7 (2018).
Fraietta, J. A. et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127, 1117–1127 (2016).
Okazaki, T., Maeda, A., Nishimura, H., Kurosaki, T. & Honjo, T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl Acad. Sci. USA 98, 13866–13871 (2001).
Davenport, A. J. et al. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol. Res. 3, 483–494 (2015).
Singh, N. et al. Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T-cell dysfunction. Cancer Discov. 10, 552–567 (2020).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Ellis, G. I., Sheppard, N. C. & Riley, J. L. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 22, 427–447 (2021).
Alfageme-Abello, O., Porret, R., Perreau, M., Perez, L. & Muller, Y. D. Chimeric antigen receptor T-cell therapy for HIV cure. Curr. Opin. HIV AIDS 16, 88–97 (2021).
Esensten, J. H., Muller, Y. D., Bluestone, J. A. & Tang, Q. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J. Allergy Clin. Immunol. 142, 1710–1718 (2018).
Haddadi, M.-H. et al. Autoimmunity as a target for chimeric immune receptor therapy: a new vision to therapeutic potential. Blood Rev. 41, 100645 (2020).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021).
Maxwell, K. G. & Millman, J. R. Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep. Med. 2, 100238 (2021).
Fang, L., Murphy, A. J. & Dart, A. M. A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Front. Pharm. 8, 186 (2017).
Aghajanian, H. et al. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433 (2019).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Pouyanfard, S. et al. Human induced pluripotent stem cell-derived macrophages ameliorate liver fibrosis. Stem Cells 39, 1701–1717 (2021).
Rurik, J. G., Aghajanian, H. & Epstein, J. A. Immune cells and immunotherapy for cardiac injury and repair. Circ. Res 128, 1766–1779 (2021).
Czechowicz, A., Kraft, D., Weissman, I. L. & Bhattacharya, D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 318, 1296–1299 (2007).
Louis, C. U. et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood, J. Am. Soc. Hematol. 113, 2442–2450 (2009).
Matthews, D. C. et al. Phase I study of 131I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood 94, 1237–1247 (1999).
Czechowicz, A. et al. Selective hematopoietic stem cell ablation using CD117-antibody–drug-conjugates enables safe and effective transplantation with immunity preservation. Nat. Commun. 10, 1–12 (2019).
Williams, JasperZ. et al. Precise T cell recognition programs designed by transcriptionally linking multiple receptors. Science 370, 1099–1104 (2020).
Roddie, C., O’Reilly, M., Dias Alves Pinto, J., Vispute, K. & Lowdell, M. Manufacturing chimeric antigen receptor T cells: issues and challenges. Cytotherapy 21, 327–340 (2019).
Levine, B. L. & June, C. H. Perspective: assembly line immunotherapy. Nature 498, S17 (2013).
Aijaz, A. et al. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2, 362–376 (2018).
Levine, B. L., Miskin, J., Wonnacott, K. & Keir, C. Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev. 4, 92–101 (2017).
Sheridan, C. Off-the-shelf, gene-edited CAR-T cells forge ahead, despite safety scare. Nat. Biotechnol. 40, 5–8 (2021).
Harrison, R. P., Zylberberg, E., Ellison, S. & Levine, B. L. Chimeric antigen receptor-T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy 21, 224–233 (2019).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
DiNofia, A. M. & Grupp, S. A. Will allogeneic CAR T cells for CD19+ malignancies take autologous CAR T cells ‘off the shelf’? Nat. Rev. Clin. Oncol. 18, 195–196 (2021).
Pfeiffer, A. et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 10, e9158 (2018).
Agarwal, S. et al. In vivo generation of CAR T cells selectively in human CD4+ lymphocytes. Mol. Ther. 28, 1783–1794 (2020).
Nawaz, W. et al. AAV-mediated in vivo CAR gene therapy for targeting human T-cell leukemia. Blood Cancer J. 11, 119 (2021).
Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use (FDA, 2020); https://www.fda.gov/media/109176/download
Posey, A. D. Jr. et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 (2016).
Yarmarkovich, M. et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 599, 477–484 (2021).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Dannenfelser, R. et al. Discriminatory power of combinatorial antigen recognition in cancer T cell therapies. Cell Syst. 11, 215–228 (2020).
Ezekian, B. et al. Contemporary strategies and barriers to transplantation tolerance. Transplantation 102, 1213–1222 (2018).
Ciurea, S. O. et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 21, 1392–1398 (2015).
Sacks, S. H. & Zhou, W. The role of complement in the early immune response to transplantation. Nat. Rev. Immunol. 12, 431–442 (2012).
Liu, C. et al. Agonistic antibody to CD40 boosts the antitumor activity of adoptively transferred T cells in vivo. J. Immunother. 35, 276–282 (2012).
Baeuerle, P. A. et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 10, 2087 (2019).
Johnson, L. A. et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 177, 6548–6559 (2006).
Oei, V. Y. S. et al. Intrinsic functional potential of NK-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol. Res 6, 467–480 (2018).
Li, Y., Hermanson, D. L., Moriarity, B. S. & Kaufman, D. S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192(2018).
Sadeghzadeh, M. et al. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 254, 117580 (2020).
Iriguchi, S. et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun. 12, 430 (2021).
Maldini, C. R., Ellis, G. I. & Riley, J. L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 18, 605–616 (2018).
Rozenbaum, M. et al. Gamma-delta CAR-T cells show CAR-directed and independent activity against leukemia. Front Immunol. 11, 1347 (2020).
Chmielewski, M. & Abken, H. CAR T cells releasing IL-18 convert to T-bet. Cell Rep. 21, 3205–3219 (2017).
Koneru, M., Purdon, T. J., Spriggs, D., Koneru, S. & Brentjens, R. J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors. Oncoimmunology 4, e994446 (2015).
Krenciute, G. et al. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 5, 571–581 (2017).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
Rupp, L. J. et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 7, 737 (2017).
Rezaei, R. et al. Combination therapy with CAR T cells and oncolytic viruses: a new era in cancer immunotherapy. Cancer Gene Ther. https://doi.org/10.1038/s41417-021-00359-9 (2021).
Fukuhara, H., Ino, Y. & Todo, T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 107, 1373–1379 (2016).
Aalipour, A. et al. Viral delivery of CAR targets to solid tumors enables effective cell therapy. Mol. Ther. Oncolytics 17, 232–240 (2020).
Hipp, S. et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 31, 1743–1751 (2017).
Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev. Pathol. 16, 223–249 (2021).
Thomas, E. D., Lochte, H. L., Cannon, J. H., Sahler, O. D. & Ferrebee, J. W. Supralethal whole body irradiation and isologous marrow transplantation in man. J. Clin. Investig. 38, 1709–1716 (1959).
Weiden, P. L. et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 300, 1068–1073 (1979).
Blazar, B. R., Murphy, W. J. & Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 12, 443–458 (2012).
Griffioen, M., van Bergen, C. A. & Falkenburg, J. Autosomal minor histocompatibility antigens: how genetic variants create diversity in immune targets. Front. Immunol. 7, 100 (2016).
Hill, G. R., Betts, B. C., Tkachev, V., Kean, L. S. & Blazar, B. R. Current concepts and advances in graft-versus-host disease immunology. Annu. Rev. Immunol. 39, 19–49 (2021).
Tivol, E., Komorowski, R. & Drobyski, W. R. Emergent autoimmunity in graft-versus-host disease. Blood 105, 4885–4891 (2005).
Wu, S. R. & Reddy, P. Tissue tolerance: a distinct concept to control acute GVHD severity. Blood 129, 1747–1752 (2017).
Zitvogel, L., Ayyoub, M., Routy, B. & Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016).
Sepich-Poore, G. D. et al. The microbiome and human cancer. Science 371, eabc4552 (2021).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Kuczma, M. P. et al. The impact of antibiotic usage on the efficacy of chemoimmunotherapy is contingent on the source of tumor-reactive T cells. Oncotarget 8, 111931 (2017).
Honda, K. & Littman, D. R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 30, 759–795 (2012).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019).
Peled, J. U. et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 382, 822–834 (2020).
Uribe-Herranz, M. et al. Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12. JCI Insight 3, e94952 (2018).
Smith, M. et al. Intestinal microbiome analyses identify biomarkers for patient response to CAR T cell therapy. Biol. Blood Marrow Transplant. 25, S177 (2019).
Smith, M., et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. (in the press).
Nguyen, C. L., Docampo, M. D., van den Brink, M. R. & Markey, K. A. The role of the intestinal microbiota in allogeneic HCT: clinical associations and preclinical mechanisms. Curr. Opin. Genet. Dev. 66, 25–35 (2021).
Acknowledgements
The authors would like to thank R. Young for insightful discussions, and the authors apologize to colleagues for work that we were unable to cite owing to space constraints. This was supported by 1P01CA214278, R01CA226983 and the Parker Institute for Cancer Immunotherapy (C.H.J.); the National Science Foundation Graduate Fellowship DGE-1321851 (A.V.F.); the National Institute of Health T32 CA009140 (T.B.); and the Go for IT Fondazione CRUI/MIUR (Italy) Fellowship 2020 (G.G.).
Author information
Authors and Affiliations
Contributions
All authors drafted, edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.H.J. has received grant support from Novartis, and has patents related to CAR therapy with royalties paid from Novartis to the University of Pennsylvania. C.H.J. is also a scientific co-founder and holds equity in Capstan Therapeutics and Tmunity Therapeutics. C.H.J. serves on the board of AC Immune and is a scientific advisor to Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Cellcarta, Celldex, Danaher, Decheng, ImmuneSensor, Poseida, Verismo, Viracta, and WIRB-Copernicus group.
Peer review
Peer review information
Nature Medicine thanks Renier Brentjens and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Karen O'Leary was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Finck, A.V., Blanchard, T., Roselle, C.P. et al. Engineered cellular immunotherapies in cancer and beyond. Nat Med 28, 678–689 (2022). https://doi.org/10.1038/s41591-022-01765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-01765-8
This article is cited by
-
Immune evasion in cell-based immunotherapy: unraveling challenges and novel strategies
Journal of Biomedical Science (2024)
-
Cystine deprivation triggers CD36-mediated ferroptosis and dysfunction of tumor infiltrating CD8+ T cells
Cell Death & Disease (2024)
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
Application of tumor microparticles in tumor prevention and treatment
Cancer Nanotechnology (2023)
-
New insights into the stemness of adoptively transferred T cells by γc family cytokines
Cell Communication and Signaling (2023)