Abstract
Background
Echocardiographic parameters of diastolic function depend on cardiac loading conditions, which are altered by positive pressure ventilation. The direct effects of positive end-expiratory pressure (PEEP) on cardiac diastolic function are unknown.
Methods
Twenty-five patients without apparent diastolic dysfunction undergoing coronary angiography were ventilated noninvasively at PEEPs of 0, 5, and 10 cmH2O (in randomized order). Echocardiographic diastolic assessment and pressure–volume-loop analysis from conductance catheters were compared. The time constant for pressure decay (τ) was modeled with exponential decay. End-diastolic and end-systolic pressure volume relationships (EDPVRs and ESPVRs, respectively) from temporary caval occlusion were analyzed with generalized linear mixed-effects and linear mixed models. Transmural pressures were calculated using esophageal balloons.
Results
τ values for intracavitary cardiac pressure increased with the PEEP (n = 25; no PEEP, 44 ± 5 ms; 5 cmH2O PEEP, 46 ± 6 ms; 10 cmH2O PEEP, 45 ± 6 ms; p < 0.001). This increase disappeared when corrected for transmural pressure and diastole length. The transmural EDPVR was unaffected by PEEP. The ESPVR increased slightly with PEEP. Echocardiographic mitral inflow parameters and tissue Doppler values decreased with PEEP [peak E wave (n = 25): no PEEP, 0.76 ± 0.13 m/s; 5 cmH2O PEEP, 0.74 ± 0.14 m/s; 10 cmH2O PEEP, 0.68 ± 0.13 m/s; p = 0.016; peak A wave (n = 24): no PEEP, 0.74 ± 0.12 m/s; 5 cmH2O PEEP, 0.7 ± 0.11 m/s; 10 cmH2O PEEP, 0.67 ± 0.15 m/s; p = 0.014; E’ septal (n = 24): no PEEP, 0.085 ± 0.016 m/s; 5 cmH2O PEEP, 0.08 ± 0.013 m/s; 10 cmH2O PEEP, 0.075 ± 0.012 m/s; p = 0.002].
Conclusions
PEEP does not affect active diastolic relaxation or passive ventricular filling properties. Dynamic echocardiographic filling parameters may reflect changing loading conditions rather than intrinsic diastolic function. PEEP may have slight positive inotropic effects.
Clinical trial registration
https://clinicaltrials.gov/ct2/show/NCT02267291, registered 17. October 2014.
Graphical abstract

Similar content being viewed by others
Background
Echocardiography is used widely to investigate cardiac function in patients on mechanical ventilation due to acute respiratory failure, cardiogenic pulmonary edema, or other critical illnesses, or for general anesthesia [1]. The echocardiographic parameters best suited for the evaluation of left or right ventricular dysfunction remain unclear. This issue has been given high priority in research agendas for critical care echocardiography [1, 2]. Diastolic dysfunction (diagnosed by echocardiography with tissue Doppler and mitral flow pattern analyses) is a frequent cause of weaning failure [3,4,5,6], has been termed “understudied” [7], and is used to guide therapeutic decision making [5, 8]. Questions remain as to whether Doppler-derived parameters truly reflect intrinsic diastolic cardiac properties or represent preload- and afterload-dependent filling phenomena [9, 10]. The positive intrathoracic pressure applied by mechanical ventilation leads to an overall preload reduction and an increase in right ventricular and decrease in left ventricular afterload [11,12,13,14]. Positive end-expiratory pressure (PEEP) influences the echocardiographic assessment of diastolic function in anesthetized and critically ill patients [15,16,17], questioning the validity of echo-Doppler assessments under mechanical ventilation. For the investigation of diastolic dysfunction independently of cardiac loading conditions, invasive pressure–volume-loop analysis with conductance catheters is the gold standard. Invasive animal studies have provided conflicting results [18, 19], and we are not aware of any reporting of invasively measured data from humans under mechanical ventilation. As the pleural space is the effective working environment of the heart, its transmural pressure may be approximated by subtracting the esophageal pressure from the intracavitary pressure [11, 20, 21].
The widespread use of positive pressure ventilation in anesthesia and critical care and the load dependency of Doppler-derived parameters for diastolic function underscore the need for the validation of echocardiographic diastology parameters measured under mechanical ventilation. With this study, we aimed to elucidate intrinsic ventricular properties under PEEP and to obtain an assembled picture of diastolic left ventricular function together with Doppler-derived filling parameters. We hypothesized that mitral inflow and tissue Doppler parameters would reflect altered cardiac loading conditions attributable to PEEP, rather than changes in myocardial properties.
Materials and methods
Participants
This prospective, controlled single-center observational study conducted at the University Hospital Bern was approved by the Ethics Committee of the Canton of Bern (KEK 104/14). Patients provided written informed consent. The report follows the STROBE guidelines. Adult patients with no known heart, pulmonary, renal, or esophageal disease scheduled for elective coronary angiography between June 2015 and January 2018 were included prospectively, unless diastolic dysfunction > grade 1 was present on transthoracic echocardiography.
Protocol
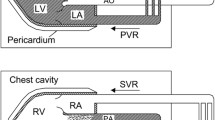
A pulmonary-artery balloon catheter was placed in the right pulmonary artery. Cardiac output was measured by thermodilution and the Fick method [22]. Left-ventricular pressure volume loops were obtained with a 7 French combined pressure-conductance catheter system (Sentron Europe BV, Roden, Netherlands and INCA; CDLeycom, Hengelo, Netherlands), calibrated as described previously [23]. An Amplatzer sizing balloon (Abbott Medical, The corporate village, Zaventeem, Belgium) was placed in the inferior vena cava for intermittent reduction of the preload (Fig. 1). Comprehensive transthoracic echocardiography (Vivid E9 and E95; GE Medical Systems, Glattbrugg, Switzerland) was performed during the pressure–volume-loop assessment. Data were stored for blinded offline analysis (EchoPac software, version 08; GE Medical Systems).
Exemplary loop family from a caval occlusion maneuver with the INCA device, recorded at a PEEP level of 10 cmH2O. The loop family corresponds to the echocardiography shown in Fig. 5
The assessment was performed with the participants breathing spontaneously, and with continuous positive airway pressure (CPAP) applied via a tight face mask; this mode of ventilation maintains continuous PEEP. Pressures of 5 and 10 cmH2O were applied in randomized order (Servo-i; Maquet, Solna, Sweden). An air-filled esophageal balloon (Nutrivent; Sidam s.r.l., Mirandola, Italy) [24, 25] was placed behind the left atrium under fluoroscopic guidance, with occlusion testing to confirm proper positioning [26, 27]. This balloon served as a surrogate for pleural pressure, which is needed for the calculation of transmural cardiac pressures. Pulmonary-artery and left-ventricular blood gases were taken at each stage of the assessment (spontaneous breathing, 5 cmH2O PEEP, and 10 cmH2O PEEP), together with noninvasive blood pressure, pulmonary artery pressure, and left-ventricular pressure–volume loops during normal respiration and expiratory holds in parallel with the echocardiographic assessment. At each stage, the cardiac preload was reduced temporarily by occlusion of the inferior vena cava to obtain pressure–volume loop families for end-systolic and end-diastolic pressure–volume relationships (ESPVR and EDPVR, respectively, Fig. 1). All invasive measurements were taken during expiratory hold to avoid additional pressure swings from respiration. Additional details are provided in Additional file 1.
Data collection, processing, and analysis
Pressure data were entered into the dedicated acquisition system with standard pressure transducers, and subsequently extracted to Matlab (Release 2018b; MathWorks, Natick, Massachusetts, USA). The isovolumetric relaxation time constant (τ) was recalculated from heartbeats during expiratory hold using exponential (τGlantz) and logistic (τlogistic) models for intraventricular and transmural ventricular pressures by subtraction of the esophageal pressure [28]. Diastole lengths were resampled to a standardized diastole length. For the intraventricular and transmural pressures, end-diastolic pressure–volume relationships were calculated using an exponential curve (P = CeβV) fit to the diastolic pressure–volume points to determine the chamber stiffness constant (β) [29] and the pressure at an end-diastolic volume of 0 ml (= C). V equals chamber volume. Additional methodological details are provided in Additional file 1.
Statistical analysis
The normality of the data distributions was assessed using the Shapiro–Wilk test and Q–Q plots. Comparisons among groups were made by one-way repeated-measures or Friedman’s analysis of variance, as appropriate, with post-hoc Bonferroni correction. ESPVRs were assessed using linear mixed-effects regression, with systolic pressure serving as the dependent variable. EDPVRs were assessed using generalized linear mixed models with a log link and gamma distribution for diastolic pressure. In all models, the volume, PEEP group, and interaction between them served as independent variables. Additionally, a random intercept and slope for volume were introduced at the participant level to allow for heterogeneity in the pressure–volume relationship among participants. For better interpretability, systolic and diastolic volumes were centered at their mean values. The analyses were performed with SPSS (version 21; IBM Corporation, Armonk, NY, USA) and Stata (version 16; StataCorp, College Station, TX, USA) software, and two-tailed p values < 0.05 were considered to be significant.
Results
Sample characteristics
30 cardiac patients (aged 18–60 years) consented to study participation. Five could not undergo assessment due to capacity constraints in the catheter laboratory. The analyses were performed with data from 25 patients (7 women, 18 men, Table 1). The sample for steady-state assessment comprised 1298 pressure–volume loops. After the exclusion of occlusion maneuver artifacts, 1598 and 1052 loops were entered into linear and generalized linear mixed-effects models for the assessment of ESPVRs and EDPVRs, respectively. The study procedures were performed without complication.
The thermodilution cardiac output decreased slightly with increasing PEEP at stable stroke volumes due to a slower heart rate with higher PEEPs (Table 2). The pulmonary artery occlusion pressure and the esophageal pressure (as a surrogate of pleural pressure) increased with PEEP. Echocardiography showed a stable mean ejection fraction of 65% ± 5% with unchanging chamber volumes (Table 2).
Invasive assessment and modeling properties
Systolic function and ventriculo-aortic coupling
The ejection fraction, end-systolic pressure, the pressure change over time (dP/dtmax, reflecting passive filling) and ESPVR slope (reflecting end-systolic elastance) did not alter with the PEEP. The aortic elastance did not change with increasing PEEP. The ratio of end-systolic to aortic elastance, an indicator of ventriculo-aortic coupling, remained unchanged with changes in PEEP, as did the stroke work and preload recruitable stroke work (Table 3). The linear mixed model revealed small, but significant, changes in the ESVPR and transmural ESPVR slopes (except for the transmural ESPVR at 5 cmH2O PEEP; Table 4). The changes in the intercepts (except for the transmural ESPVR at 10 cmH2O PEEP) were also small but significant.
Relaxation
The application of PEEP significantly increased τ values for intracavitary ventricular pressures, indicating slower early relaxation of the left ventricle. This effect could be observed independently of the decay model used, and was sustained for the transmural cardiac pressures (Fig. 2, Table 5). When τ was corrected for the diastole length, the effect disappeared for the transmural pressure decay.
Passive filling properties
Early and mid-diastolic ventricular pressures and simultaneous pulmonary-artery occlusion pressures were significantly higher with PEEP (5 and 10 cmH2O) than without PEEP, with no difference between PEEP levels (Table 6, Fig. 3a). This effect disappeared when transmural pressures were calculated (Table 6, Fig. 2b). The instantaneous transmural trans-mitral pressure gradient remained unchanged with PEEP (Fig. 2).
a Left ventricular filling curves represented by diastolic ventricular pressure and PAOP, with 95% confidence intervals as dashed lines. The data were resampled for a filling period of 125 samples (500 ms). The dotted lines for early, mid- and late diastolic data points indicate the data presented in Table 4. b Transmural left ventricular filling curves Means with 95% confidence intervals as dashed lines are presented. The data were resampled to a filling period of 125 samples (500 ms). The dotted lines for early, mid- and late diastolic data points. PAOP pulmonary-artery occlusal pressure, LVP left ventricular pressure, PEEP positive end-expiratory pressure
Positions of the EDPVR and chamber stiffness
The generalized linear mixed-effects model revealed small, but significant, changes in the natural logarithms of the intercept changes in the absolute and transmural EDPVRs. The resulting intercept pressures from the inverse function (for intracavitary pressures: no PEEP, 9.57 mmHg; 5 cmH2O PEEP, 10.31 mmHg; 10 cmH2O PEEP, 12.57 mmHg; for transmural pressures, no PEEP 4.73 mmHg, 5 cmH2O PEEP, 3.82 mmHg; 10 cmH2O PEEP, 3.68 mmHg) indicate a downward shift of the EDPVR for the transmural pressure, with a resolution of between-PEEP differences. The change in slope, representing the chamber stiffness, lost significance when corrected for transmural pressure (Table 7). The shift in position and change in stiffness with increasing PEEP disappeared when transmural pressures were considered (Fig. 4).
a End-systolic pressure–volume relationships (ESPVRs) for 0, 5, and 10 cmH2O PEEP, according to the mixed linear model equations in Table 4. The equation values are reported at the mean centered volume (50.4 ml), indicated by the dotted line. b End-diastolic pressure–volume relationships (EDPVRs), according to the mixed-effect generalized linear model equations in Table 7. The equation values are reported at the mean centered volume (136.2 ml), indicated by the dotted line. PEEP positive end-expiratory pressure
Echocardiographic diastole evaluation
Trans-mitral flow velocities
The peak early and late trans-mitral flow velocities (E and A) decreased significantly with increasing PEEP, with no change in the E/A ratio. The duration of the E wave increased in absolute terms, but remained constant relative to the duration of diastole and the full cardiac cycle. The duration of the A wave and the deceleration time remained constant (Table 5, Fig. 5).
Exemplary echocardiography tracings at a PEEP level of 10 cm H2O. The frames that correspond to the PV-Loop family in Fig. 1 are shown. The left panel shows a transmitral pulsed wave Doppler trace, the right panel a tissue Doppler tracing
Tissue Doppler properties of the mitral annulus
The peak early septal tissue velocity (Eʹ) decreased with PEEP. The lateral Eʹ and late tissue velocities (Aʹ) were less affected. The isovolumetric relaxation time increased with PEEP (Table 8).
Discussion
The main finding of this study is that the increase in intrathoracic pressure caused by mechanical ventilation had no intrinsic influence on cardiac function, assessed invasively by the gold standard (impedance catheter). Inotropy, represented by the ESPVR, increased slightly with PEEP. The invasive assessment of diastolic function revealed a small influence of PEEP on active relaxation, with an apparent increase in τ, upward shift of the EDPVR, and decreasing chamber stiffness, indicating altered passive ventricular filling and elastic properties, but only when the pressures were referenced to atmosphere [11]. These changes in invasive measures disappeared with the measurement of transmural chamber pressures, which reflect the working condition of the left ventricle. These results stand in contrast to echocardiographic parameters, as modest PEEP influences the transmitral flow pattern and annular tissue Doppler properties.
These findings challenge current paradigms and must be placed in clinical and physiological contexts. The inaccuracy of transmitral indices for the assessment of diastolic dysfunction has long been discussed [9] 30. Comparative Doppler-conductance catheter studies with non-ventilated patients have shown that tissue Doppler indices may better reflect diastolic dysfunction than do transmitral flow velocities, and that increasing E/E’ ratios are the best indicators of diastolic dysfunction [30, 31]. Our data, support this notion. The load dependencies of transmitral E and A waves are recognized [17], albeit not in comparison with invasive assessment under positive-pressure ventilation. Juhl-Ohlsen and colleagues . [15, 16] demonstrated the influence of PEEP on E and A waves in anesthetized cardiac patients. PEEP increases the pleural pressure [20], explaining its well-established preload-reducing effect [11, 21, 25, 32]. Our echocardiographic findings are in line with these previous observations. The E/Eʹ ratio, strongly predictive of ventilator weaning failure [3], was not affected by PEEP in our population.
We observed apparent slowing of active relaxation, with increases in τ with the application of PEEP. Despite its significance in the intracavitary pressure assessment, the magnitude of this change appears to be clinically marginal. For its proper interpretation, two physiological phenomena need to be taken into account. The first is the heart rate, and thus the duration of diastole. Several studies have shown that τ declines with increasing heart rate and vice versa [33,34,35]. As the heart rate changes with PEEP, we normalized the diastole duration in a second model of τ to exclude influences of changing diastole length in this study. The second issue is the exposure of the heart to pleural, rather than atmospheric, pressure [11]. In routine cardiac catheterization and hemodynamic monitoring, intracavitary and intravascular pressures are measured with a zero reference to the atmosphere. The pericardial pressure, however, approximates the pleural pressure [36]. Cardiac working conditions are better represented by transmural rather than intracavitary pressures. The subtraction of the esophageal pressure from intracavitary pressures may serve as a sufficient approximation [11, 20, 36,37,38]. When this intrathoracic (i.e., pleural) pressure reference is taken into account, the apparent effect of PEEP on τ disappears. Thus, findings may reflect the use of an inappropriate pressure reference, rather than the occurrence of a true physiological phenomenon. The consistency of the findings obtained with various modeling approaches for τ reflects reliability, particularly as the logistic modeling of pressure decay is relatively resistant to respiration influences [28]. In conductance volumetry, unchanged ventricular volumes may exclude ventricular interdependence as a cause of impaired relaxation [39].
PEEP applies a mechanical constraint to the heart by increasing the pleural pressure and elevating the functional residual capacity of the lung, with compression of the cardiac fossa [40]. Increased pericardial pressure shifts the pressure–volume curve upward [41], and a similar shift in the EDPVR slope was observed in this study. This upward shift, and the apparent decrease in chamber stiffness, disappeared when transmural pressures were considered. These findings may thus be considered artifacts of the use of the atmospheric pressure reference. The clinically modest PEEP levels used in this study had no apparent effect on cardiac chamber volumes; shifts may differ with the application of greater pressure, particularly with invasive mechanical ventilation [42]. We may only speculate on the effects of higher PEEP, as the preload reducing effect together with mechanical constraint may dominate afterload reduction and resemble restrictive filling with signs of obstructive shock [11]. Such data would need to be gained from invasive ventilation because higher pressure levels on NIV are usually badly tolerated.
The slope of the ESPVR at 10 cm H2O PEEP increased, with a positive (intracavitary) or no (transmural) relevant increase in the pressure intercept, in this study. These findings are consistent with an increased inotropic state of the left ventricle [29]. At 5 cmH2O PEEP, the increase in slope was less prominent and of borderline significance. We have no mechanistically convincing explanation for this unexpected result. Data from animal studies suggest that PEEP negatively affects the coronary blood flow [43], which would have the opposite effect as we observed. The Anrep effect, or slow force response to increased stretch, could explain the increased inotropy [44, 45], but the afterload seems to be constant in our context with stable ventriculo-arterial coupling across PEEP levels. Verification of the direct positive inotropic effects of PEEP would provide an additional mechanistic explanation of the beneficial effects of positive pressure ventilation for acute cardiac failure, beyond the classic concept of preload and afterload reduction.
Limitations
Several limitations of this study must be considered. First, the study participants were cardiac patients with no apparent diastolic dysfunction at the time of enrollment, as verified by echocardiographic grading and low τ values on spontaneous breathing. Whether similar results would be obtained in patients with such dysfunction or those under invasive mechanical ventilation in an intensive care unit setting warrants further investigation. The advantage of conducting the study with patients without diastolic dysfunction is that we could demonstrate the appearance of such dysfunction with echocardiography. We can only speculate about effects of PEEP in patients with preexisting diastolic function. These effects may be less unidirectional, since the mechanical constraint caused by PEEP may be counterbalanced by its afterload reducing effect in the clinical context of heart failure with preserved ejection fraction or obstructive sleep apnea. Clinical studies indicate long term amelioration of diastolic dysfunction with positive pressure ventilation [46, 47].
Second, our echocardiographic finding of increasing diastolic dysfunction severity with increasing airway pressure may be clinically elusive. Its importance lies not in its magnitude, but in its difference from the conductance-catheter gold standard. Third, voluntary control of respiration in an awake person under an invasive hemodynamic study is demanding. We took meticulous care to instruct the participants in performing expiratory holds and monitored their respiratory drives with esophageal pressure swings. An attending intensivist supervised all maneuvers, and the esophageal pressure swings were screened for inadvertent Valsalva maneuvers. Still, unintended respiratory variation cannot be excluded. Thus, the use of τ logistic, which is least influenced by respiration [28], was of particular value. Fourth, an esophageal balloon is the only feasible pleural pressure surrogate in an awake patient and its use is recommended [38], although this technique may not directly reflect pericardial pressure. We have experience in esophageal balloon use [20, 25], and calibrated the balloon using an accepted technique in this study [37, 38]. As each patient served as his/her own control in the randomized crossover setting, the esophageal pressure was a valid relative reference, although absolute values might have been slightly inaccurate. Fifth, calibration of the PV loops required a two-step approach. The stroke volume was calibrated via thermodilution at every PEEP level. We followed a standardized approach for thermodilution by averaging the three closest cardiac output measurements from five saline injections [48]. The PV loops were positioned on the volume axis by calculating the end-diastolic volume from a baseline ejection fraction measurement (obtained by echocardiography or with hypertonic saline injection). The variability of the PV loop position through the ejection fraction may have contributed to shifts in intercept and slope, but was partially accounted for with the use of mixed-effects models for the ESPVR and EDPVR. Last, our sample size could not be based on a power calculation, because of the exploratory nature of this study and the lack of preexisting data.
Conclusion
We conclude that dynamic echocardiographic filling parameters reflect changing loading conditions, rather than diastolic function, under the application of positive airway pressure. Furthermore, invasive assessments should be referenced to intrathoracic pressure conditions, rather than to atmospheric pressure. The role of echocardiography in the detection of diastolic dysfunction in mechanically ventilated patients needs to be reassessed.
Availability of data and materials
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- A:
-
Peak late mitral inflow velocity
- CPAP:
-
Continuous positive airway pressure
- DT:
-
Deceleration time
- E:
-
Peak early mitral inflow velocity
- E’:
-
Peak early diastolic mitral annular velocity
- Ea:
-
Arterial elastance
- Ees:
-
End-systolic elastance
- EDPVR:
-
End-diastolic pressure–volume relationship
- EDV:
-
End-diastolic volume
- EDP:
-
End-diastolic pressure
- EF:
-
Ejection fraction
- ESP:
-
End-systolic pressure
- ESPVR:
-
End-systolic pressure–volume relationship
- ESV:
-
End-systolic volume
- IVRT:
-
Isovolumic relaxation time
- LV:
-
Left ventricle
- LVEDP:
-
Left ventricular end-diastolic pressure
- PAOP:
-
Pulmonary artery occlusion pressure
- PEEP:
-
Positive end-expiratory pressure
- PFR:
-
Peak filling rate
- PW:
-
Pulsed wave Doppler mode
- SV:
-
Stroke volume
- TAPSE:
-
Tricuspid annular plane systolic excursion
- TDI:
-
Tissue Doppler imaging
References
Vieillard-Baron A, Millington SJ, Sanfilippo F, Chew M, Diaz-Gomez J, McLean A, Pinsky MR, Pulido J, Mayo P, Fletcher N (2019) A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 45(6):770–788
Mayo P, Arntfield R, Balik M, Kory P, Mathis G, Schmidt G, Slama M, Volpicelli G, Xirouchaki N, McLean A et al (2017) The ICM research agenda on critical care ultrasonography. Intensive Care Med 43(9):1257–1269
Papanikolaou J, Makris D, Saranteas T, Karakitsos D, Zintzaras E, Karabinis A, Kostopanagiotou G, Zakynthinos E (2011) New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med 37:1976
Lamia B, Maizel J, Ochagavia A, Chemla D, Osman D, Richard C, Teboul JL (2009) Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med 37(5):1696–1701
Caille V, Amiel JB, Charron C, Belliard G, Vieillard-Baron A, Vignon P (2010) Echocardiography: a help in the weaning process. Crit Care (London, England) 14(3):R120
Moschietto S, Doyen D, Grech L, Dellamonica J, Hyvernat H, Bernardin G (2012) Transthoracic Echocardiography with Doppler Tissue Imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care (London, England) 16(3):R81
Saleh M, Vieillard-Baron A (2012) On the role of left ventricular diastolic function in the critically ill patient. Intensive Care Med 38(2):189–191
Routsi C, Stanopoulos I, Kokkoris S, Sideris A, Zakynthinos S (2019) Weaning failure of cardiovascular origin: how to suspect, detect and treat-a review of the literature. Ann Intensive Care 9(1):6
Maurer MS, Spevack D, Burkhoff D, Kronzon I (2004) Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol 44(8):1543–1549
Aurigemma GP, Zile MR, Gaasch WH (2004) Lack of relationship between Doppler indices of diastolic function and left ventricular pressure transients in patients with definite diastolic heart failure. Am Heart J 148(3):E12
Grubler MR, Wigger O, Berger D, Blochlinger S (2017) Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med Week 147:14491
Pinsky MR, Matuschak GM, Klain M (1985) Determinants of cardiac augmentation by elevations in intrathoracic pressure. J Appl Physiol (Bethesda, Md: 1985) 58(4):1189–1198
Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD (1995) Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 91(6):1725–1731
Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F (1999) Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol (Bethesda, Md: 1985) 87(5):1644–1650
Juhl-Olsen P, Frederiksen CA, Hermansen JF, Jakobsen CJ, Sloth E (2012) Echocardiographic measures of diastolic function are preload dependent during triggered positive pressure ventilation: a controlled crossover study in healthy subjects. Crit Care Res Pract 2012:703196
Juhl-Olsen P, Hermansen JF, Frederiksen CA, Rasmussen LA, Jakobsen CJ, Sloth E (2013) Positive end-expiratory pressure influences echocardiographic measures of diastolic function: a randomized, crossover study in cardiac surgery patients. Anesthesiology 119(5):1078–1086
Quintard H, Muller L, Philip I, Lena P, Ichai C (2012) Influence of acute preload changes on mitral annulus velocity measured by tissue Doppler echocardiography in critically ill patients. J Clin Ultrasound 40(7):419–423
Fewell JE, Abendschein DR, Carlson CJ, Rapaport E, Murray JF (1981) Continuous positive-pressure ventilation does not alter ventricular pressure-volume relationship. Am J Physiol 240(6):H821-826
Haynes JB, Carson SD, Whitney WP, Zerbe GO, Hyers TM, Steele P (1980) Positive end-expiratory pressure shifts left ventricular diastolic pressure-area curves. J Appl Physiol Respir Environ Exerc Physiol 48(4):670–676
Berger D, Bloechlinger S, Takala J, Sinderby C, Brander L (2014) Heart-lung interactions during neurally adjusted ventilatory assist. Crit Care (London, England) 18(5):499
Berger D, Takala J (2018) Determinants of systemic venous return and the impact of positive pressure ventilation. Ann Transl Med 6(18):350
LaFarge CG, Miettinen OS (1970) The estimation of oxygen consumption1. Cardiovasc Res 4(1):23–30
Steendijk P, Staal E, Jukema JW, Baan J (2001) Hypertonic saline method accurately determines parallel conductance for dual-field conductance catheter. Am J Physiol Heart Circ Physiol 281(2):H755-763
Chiumello D, Gallazzi E, Marino A, Berto V, Mietto C, Cesana B, Gattinoni L (2011) A validation study of a new nasogastric polyfunctional catheter. Intensive Care Med 37(5):791–795
Berger D, Moller PW, Weber A, Bloch A, Bloechlinger S, Haenggi M, Sondergaard S, Jakob SM, Magder S, Takala J (2016) Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am J Physiol Heart Circ Physiol 311(3):H794-806
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126(5):788–791
Baydur A, Cha EJ, Sassoon CS (1987) Validation of esophageal balloon technique at different lung volumes and postures. J Appl Physiol 62(1):315–321
Ogilvie LM, Edgett BA, Huber JS, Platt MJ, Eberl HJ, Lutchmedial S, Brunt KR, Simpson JA (2020) Hemodynamic assessment of diastolic function for experimental models. Am J Physiol Heart Circ Physiol 318(5):H1139-h1158
Burkhoff D, Mirsky I, Suga H (2005) Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289(2):H501-512
Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M et al (2007) Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 116(6):637–647
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794
Moller PW, Winkler B, Hurni S, Heinisch PP, Bloch A, Sondergaard S, Jakob SM, Takala J, Berger D (2017) Right atrial pressure and venous return during cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 313(2):H408-h420
Frederiksen JW, Weiss JL, Weisfeldt ML (1978) Time constant of isovolumic pressure fall: determinants in the working left ventricle. Am J Physiol 235(6):H701-706
Shen W, Xu X, Lee T-F, Schmölzer G, Cheung P-Y (2019) The relationship between heart rate and left ventricular isovolumic relaxation during normoxia and hypoxia-asphyxia in newborn piglets. Front Physiol 10(525)
Weiss JL, Frederiksen JW, Weisfeldt ML (1976) Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Investig 58(3):751–760
Repessé X, Vieillard-Baron A, Geri G (2018) Value of measuring esophageal pressure to evaluate heart-lung interactions-applications for invasive hemodynamic monitoring. Ann Transl Med 6(18):351
Pinsky MR (2014) Why knowing the effects of positive-pressure ventilation on venous, pleural, and pericardial pressures is important to the bedside clinician?*. Crit Care Med 42(9):2129–2131
Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S et al (2016) Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 42(9):1360–1373
Kasner M, Westermann D, Steendijk P, Drose S, Poller W, Schultheiss HP, Tschope C (2012) Left ventricular dysfunction induced by nonsevere idiopathic pulmonary arterial hypertension: a pressure-volume relationship study. Am J Respir Crit Care Med 186(2):181–189
Pinsky MR (2012) Heart lung interactions during mechanical ventilation. Curr Opin Crit Care 18(3):256–260
Tyberg JV, Misbach GA, Glantz SA, Moores WY, Parmley WW (1978) A mechanism for shifts in the diastolic, left ventricular, pressure-volume curve: the role of the pericardium. Eur J Cardiol 7(Suppl):163–175
Veddeng OJ, Myhre ES, Risøe C, Smiseth OA (1992) Selective positive end-expiratory pressure and intracardiac dimensions in dogs. J Appl Physiol (Bethesda, Md: 1985) 73(5):2016–2020
Fessler HE, Brower RG, Wise R, Permutt S (1990) Positive pleural pressure decreases coronary perfusion. Am J Physiol 258(3 Pt 2):H814-820
Cingolani HE, Perez NG, Cingolani OH, Ennis IL (2013) The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol 304(2):H175-182
Dowrick JM, Tran K, Loiselle DS, Nielsen PMF, Taberner AJ, Han J-C, Ward M-L (2019) The slow force response to stretch: controversy and contradictions. Acta Physiol 226(1):e13250
D’Elia E, Ferrero P, Vittori C, Iacovoni A, Grosu A, Gori M, Duino V, Perlini S, Senni M (2019) Beneficial effects of adaptive servo-ventilation on natriuretic peptides and diastolic function in acute heart failure patients with preserved ejection fraction and sleep-disordered breathing. Sleep Breath 23(1):287–291
Masa JF, Mokhlesi B, Benítez I, Mogollon MV, Gomez de Terreros FJ, Sánchez-Quiroga M, Romero A, Caballero-Eraso C, Alonso-Álvarez ML, Ordax-Carbajo E et al (2020) Echocardiographic changes with positive airway pressure therapy in obesity hypoventilation syndrome. Long-term pickwick randomized controlled clinical trial. Am J Respir Crit Care Med 201(5):586–597
Jansen JR (1995) The thermodilution method for the clinical assessment of cardiac output. Intensive Care Med 21(8):691–697
Acknowledgements
We thank our study nurse Michael Lensch for his excellent technical support.
Funding
Open access funding provided by University of Bern. This study was funded by two unrestricted research grants from the Gottfried und Julia Bangerter-Rhyner Stiftung (Bern, Switzerland) and an unrestricted research grant from the Swiss Heart Foundation (Bern, Switzerland), all awarded to Stefan Bloechlinger and David Berger.
Author information
Authors and Affiliations
Contributions
DB, OW, MG, and SB conceived and designed the study, and conducted the experiments and data collection and analysis. AB conducted the experiments and data collection. SDM and RK performed the echocardiography and data collection. KFB analyzed the data. DB, OW, OS, KFB, and SB performed the statistical analysis. DB and OW drafted the manuscript. All authors contributed to manuscript revision and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The Department of Intensive Care Medicine of the Inselspital has, or has had in the past, research contracts with Abionic SA, AVA AG, CSEM SA, Cube Dx GmbH, Cyto Sorbents Europe GmbH, Edwards Lifesciences LLC, GE Healthcare, ImaCor Inc., MedImmune LLC, Orion Corporation, and Phagenesis Ltd.; and research and development/consulting contracts with Edwards Lifesciences LLC, Nestec SA, and Wyss Zurich. The money was paid into a departmental fund; Drs. Berger, Bloch, and Bachmann received no personal financial gain and declare that they have no personal conflict of interest. The Department of Intensive Care Medicine of the Inselspital has received unrestricted educational grants from the following entities for the organization of a quarterly postgraduate educational symposium, the Berner Forum for Intensive Care (until 2015): Abbott AG, Anandic Medical Systems, Astellas, AstraZeneca, Bard Medica SA, Baxter, B | Braun, CSL Behring, Covidien, Fresenius Kabi, GSK, Lilly, Maquet, MSD, Novartis, Nycomed, Orion Pharma, Pfizer, and Pierre Fabre Pharma AG (formerly known as RobaPharm). It has received unrestricted educational grants from the following entities for the organization of biannual postgraduate courses in the fields of critical care ultrasound and the management of ECMO and mechanical ventilation: Abbott AG, Anandic Medical Systems, Bard Medica SA., Bracco, Dräger Schweiz AG, Edwards Lifesciences AG, Fresenius Kabi (Schweiz) AG, Getinge Group Maquet AG, Hamilton Medical AG, Pierre Fabre Pharma AG (formerly known as RobaPharm), PanGas AG Healthcare, Pfizer AG, Orion Pharma, and Teleflex Medical GmbH. Drs. Bloechlinger, Wigger, De Marchi, Kurmann, Stalder, and Grübler have no conflict of interest to declare.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Canton of Bern (KEK 104/14). Patients provided written informed consent prior to enrollment.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berger, D., Wigger, O., de Marchi, S. et al. The effects of positive end-expiratory pressure on cardiac function: a comparative echocardiography-conductance catheter study. Clin Res Cardiol 111, 705–719 (2022). https://doi.org/10.1007/s00392-022-02014-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02014-1