Abstract
Background: Stent underexpansion increases the risk of cardiac adverse events. At present, there are limited options to treat refractory stent underexpansion. In this context, the intravascular lithotripsy (IVL) system might be a safe and effective strategy.
Aims: We aimed to evaluate the safety and efficacy of IVL in addressing resistant stent underexpansion due to heavy underlying calcification.
Methods: This was an international multicentre registry including patients receiving IVL therapy to treat stent underexpansion from December 2017 to August 2020. Angiographic and intracoronary imaging data were collected. The efficacy endpoint was device success (technical success with a final percentage diameter stenosis <50%). The safety endpoint was in-hospital major adverse cardiac events (MACE).
Results: Seventy patients were included, the mean age was 73±9.2 years and 76% were male. The median time from stent implantation to IVL therapy was 49 days (0-2,537). Adjuvant treatment with non-compliant balloon dilatations pre- and post-IVL was performed in 72.3% and 76.8% of patients, respectively, and additional stenting was performed in 22.4%. Device success was 92.3%. Minimum lumen diameter increased from 1.49±0.73 mm to 2.41±0.67 mm (p<0.001) and stent expansion increased by 124.93±138.19% (p=0.016). No IVL-related procedural complications or MACE were observed. The use of bailout IVL therapy directly after stenting and the presence of ostial underexpanded lesions negatively predicted lumen diameter gain.
Conclusions: Coronary lithotripsy is safe and effective in increasing lumen and stent dimensions in underexpanded stents secondary to heavily calcified lesions.
Introduction
Coronary artery calcification complicates stent delivery and stent expansion, and increases the risk of vessel perforation during percutaneous coronary intervention (PCI)1. Stent underexpansion (SU) is a strong predictor of restenosis and stent thrombosis23. Therefore, appropriate plaque modification prior to stent implantation is essential in order to allow adequate device deployment. At present, there are limited options to treat underexpanded stents beyond prolonged dilatations with high- and super high-pressure non-compliant (NC) balloons. Rotational and orbital atherectomy (RA, OA) and excimer laser are off-label options with variable rates of success and high risk of procedural complications456. Recently, the use of intravascular lithotripsy (IVL), both for previously implanted stents as well as a bailout strategy directly after stent implantation in case of resistant underexpansion, has been postulated as a potential alternative with encouraging results.
The IVL system (Shockwave Medical) consists of a rapid exchange semi-compliant balloon catheter with integrated lithotripsy electrodes and an electrical pulse generator that produces unfocused circumferential mechanical energy to crack calcified plaques78. In de novo coronary artery calcified lesions, IVL therapy demonstrated high procedural success, few procedural complications and few adverse clinical outcomes9101112. However, data on the off-label use of IVL in the setting of resistant SU are limited to anecdotal case reports1314151617.
The present registry aimed to investigate the safety and efficacy of IVL in the treatment of resistant SU due to underlying calcific plaques.
Methods
Population
This was an international multicentre registry including all patients who underwent IVL therapy to treat significant SU in previously implanted stents or as a bailout strategy in immediately implanted stents, from December 2017 to August 2020, in 7 centres in the European Union and Canada. The indication and timing of IVL therapy, the use of pre- and post-IVL dilatation with NC balloons, additional stenting, and the concomitant use of intracoronary imaging to guide the procedure were at the discretion of the operator.
Data collection and analysis
Clinical data, procedure-related characteristics, angiographic and intracoronary imaging data, and follow-up information until discharge were anonymised and transferred to a centralised core lab in the Erasmus University Medical Center for further analysis.
Offline quantitative coronary analysis (QCA) was performed by a dedicated software, CAAS 8.0 (Pie Medical Imaging). Measurements included reference stent diameter (RSD), maximum, mean and minimum lumen diameters (MaxLD, MeanLD and MLD, respectively), and percentage diameter stenosis (%DS).
Both, intravascular ultrasound (IVUS) and optical coherence tomography (OCT) analyses were performed offline using QCU-CMS 4.69 software (Leiden University Medical Centre). Pre- and post-PCI pullbacks were matched by identifying branches, vessel ostium, stent borders and/or other trackable features to obtain the following parameters: reference vessel area (RVA), maximum, mean and minimum lumen area (MaxLA, MeanLA and MLA, respectively), and minimum stent area (MSA). Percentage of lumen area stenosis (%LA stenosis) was calculated following the formula: ([RVA-MLA]/RVA)*100. MLA and MSA were defined as the smallest lumen and stent areas within the target stented segment. Stent expansion (SE) was calculated following the formula: (MSA/RVA)*100. Matched MLA in post-PCI pullbacks was defined as the lumen area measured at the same site of the pre-PCI MLA. Stent eccentricity index (EI) was calculated as the ratio between the minimum and maximum diameter at the site of the MLA and the matched MLA in the post-PCI pullbacks.
Endpoints definitions
The primary efficacy endpoint was device success, a composite of technical success (successful lesion crossing + successful delivery of intended number of IVL pulses + successful retrieval of the device) and residual %DS <50% as assessed by offline QCA analysis. The primary safety endpoint was in-hospital major adverse cardiac events (MACE), defined as a composite of cardiac death, non-fatal myocardial infarction and any repeat revascularisation18. Secondary endpoints included procedural success (defined as device success in the absence of MACE until discharge) and technical success.
Clinical follow-up
All patients were followed up until discharge. Clinical events were adjudicated by the treating physicians and collected from the patient’s medical records.
Statistical analysis
Categorical variables are presented as fractions and percentages. Continuous variables are presented as mean±standard deviation (SD) if normally distributed; otherwise, continuous variables are presented as median (25th-75th percentile). Paired comparisons of continuous values between pre- and post-IVL therapy were performed using the paired t-test if normally distributed, otherwise the Wilcoxon signed-rank test was used.
Associations between clinical and procedural characteristics and lumen diameter gain were investigated using univariable linear regression. Assumptions of normality and homoscedasticity were checked by drawing scatterplots of predicted values versus residual values, as well as normal probability plots. After log-10 transformation of the variable “lumen diameter gain”, assumptions were satisfied.
Based on clinical considerations, the variables “bailout IVL therapy directly after stenting”, “additional stenting”, “intracoronary imaging-guided procedure”, “multiple stent layers”, “post-IVL dilatation with NC balloons ≥1:1 ratio”, “underexpanded segment length ≥20 mm” and “ostial location of the target stented segment” were entered into a multivariable model in order to adjust for their effect as potential confounders. Results of the linear regression model are presented as standardised coefficients (beta) with 95% confidence intervals (95% CI). Two-tailed p-values below 0.05 were considered statistically significant. Statistical analysis was conducted using SPSS for Windows version 25 (IBM).
Results
A total of 70 patients were included. The mean age was 73±9.2 years and 53 (75.7%) were male. The indication for PCI was acute coronary syndrome in 37 (52.8%) cases (10% presented with ST-segment elevation myocardial infarction [STEMI]). The left anterior descending coronary artery was the target vessel in 36 (51.4%) patients and the left main was involved in 11 (15.7%) cases. The type of stent was known in 55 (78.6%) cases, of which 54 (98.1%) had an underexpanded metallic drug-eluting stent (DES). In 29 (41.4%) cases IVL therapy was applied as a bailout strategy after stent deployment to improve SU. Multiple stent layers (at least two layers of stent as a result of repeat stenting to treat prior stent failure) were present in 15 (21.4%) cases (Table 1).
The median (25th-75th percentile) IVL balloon diameter was 3.5 mm (3.0-3.9 mm) and a median of 80 (80-80) lithotripsy pulses were applied per vessel, with only 1 coronary artery per patient receiving IVL therapy. Pre- and post-IVL dilatation with NC balloons, sized in a ratio ≥1:1 with respect to the stent nominal size, were used in 37 (69.8%) and 41 (77.4%) cases, respectively, and 15 (22.4%) patients received additional stenting. In 68 (97.1%) patients, post-procedural final Thrombolysis in Myocardial Infarction (TIMI) 3 flow was achieved, and in 2 patients final TIMI flow was 0 (1 case following unsuccessful crossing of a chronic total occlusion of the target segment and in the 2nd case a no-reflow phenomenon was observed that was considered unrelated to the use of IVL) (Table 2).
Offline paired QCA data analysis revealed a significant gain in lumen diameter from 1.49±0.73 mm to 2.41±0.67 mm (p<0.001) with a significant reduction in %DS of 57.08±26.85% (p<0.001) (Table 3).
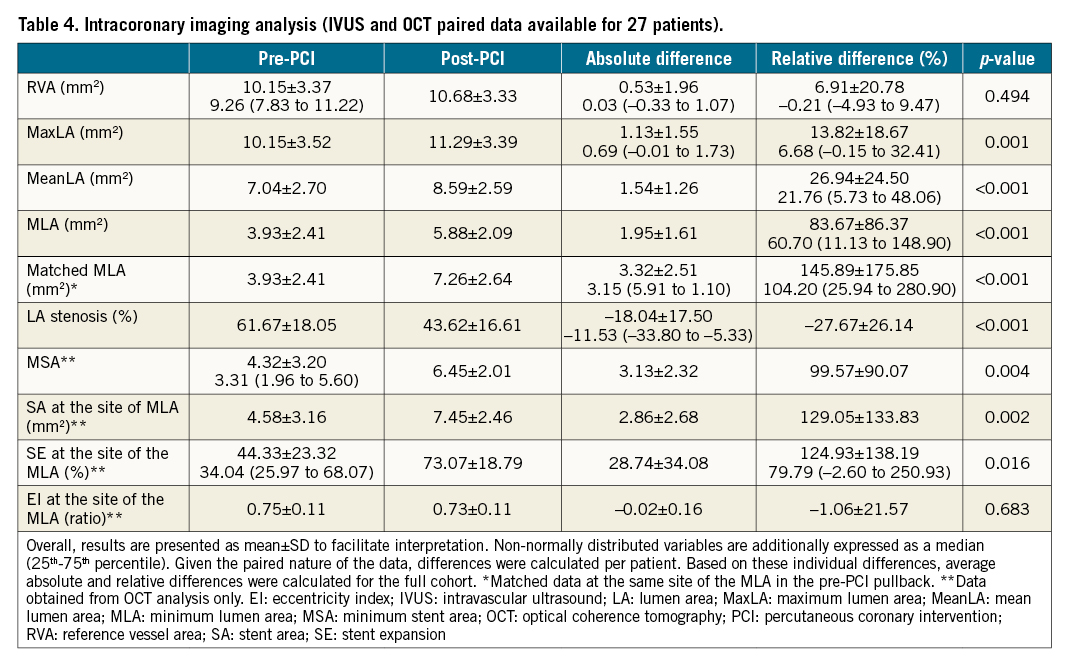
Intracoronary imaging was used to guide the procedure in 44 (62.9%) cases. Paired data analysis demonstrated a significant gain in MLA from 3.93±2.41 mm2 to 5.88±2.09 mm2 (p<0.001), supported by a significant increase in MSA from 4.32±3.20 mm2 to 6.45±2.01 mm2 (p<0.004). When taking into account the exact location of the MLA present in the pre-PCI pullbacks (matched areas), both lumen area and SE increased significantly, by 145.89±175.85% (p<0.001) and 124.93±138.19 (p=0.016), respectively (Figure 1, Table 4).
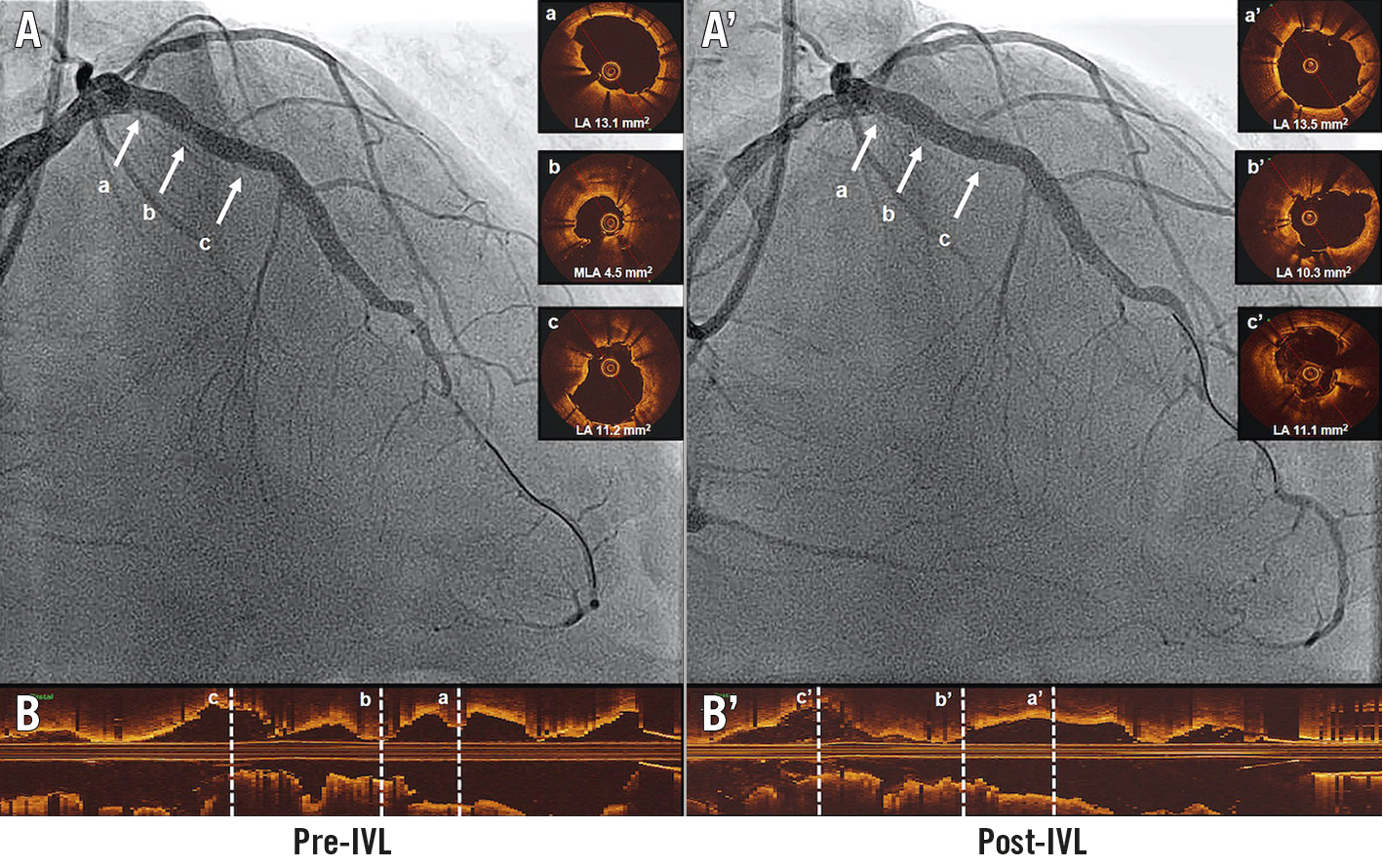
Figure 1. Intravascular coronary lithotripsy for the treatment of stent underexpansion. A. Angiographic view pre-IVL. B. Optical coherence tomography longitudinal view pre-IVL. (a) Proximal stent reference. (b) Minimum lumen area. (c) Distal stent reference. A’. Angiographic view post-IVL. B’. Optical coherence tomography longitudinal view post-IVL. (a’) Proximal stent reference. (b’) Matched minimum lumen area. (c’) Distal stent reference. IVL: intravascular lithotripsy
Median hospital stay was 2 (1-5) days and no MACE were reported. Two patients died of non-cardiac causes. No procedural complications resulted from the use of the IVL system. Device success and procedural success were both 92.3%, and technical success was 95.7%. The IVL balloon reached the target lesion in 69 (98.6%) cases (in 1 patient the balloon failed to cross the lesion, and no therapy was administered). In 2 patients (3%), the IVL balloon ruptured before completing the intended number of pulses (Supplementary Table 1).
In multivariable analysis, both bailout IVL therapy directly after stenting (β -0.49, 95% CI: -0.52 to -0.10; p=0.004) and ostial location of the target stented segment (β -0.39, 95% CI: -0.59 to -0.07; p=0.013) negatively impacted lumen diameter gain (Supplementary Table 2).
Discussion
The international multicentre CRUNCH registry is the largest study to date examining the safety and efficacy of IVL therapy for the treatment of resistant SU. Our results can be summarised as follows: 1) the IVL system had a high rate of device and technical success, with significant gains in lumen and stent areas; 2) bailout IVL therapy directly after stent implantation and ostial location of the target stented segment negatively impacted further lumen diameter gain; and 3) the IVL system was safe, with no device-related procedural complications or in-hospital adverse events.
The previous DISRUPT CAD I, II and III studies demonstrated 100% efficacy in achieving a residual DS <50% after lithotripsy in calcified native coronary arteries91011. Our results extend these findings to the setting of prior stented coronary segments and showed that IVL therapy can reach that goal in 97% of the cases, accounting for an overall device success of 92.3%. Of note, in 53% of our cases, a residual diameter stenosis <20% was reached, a threshold typically reserved to evaluate the effectiveness of coronary intervention devices for the treatment of stenosis in native coronary arteries19.
At present, limited alternative options are available to treat resistant SU refractory to high-pressure balloon dilatation. In the ELEMENT registry (n=28), the use of contrast-enhanced excimer laser was shown to increase MSA >1 mm2 in 96.4% of the cases, and a 98% technical success rate was demonstrated with the use of OA in a series of 41 patients620. RA of previous stented segments, also known as “stentablation”, successfully reduced diameter stenosis (<30-50%) in 92-100% of the cases2122. However, burr upsizing (up to 2/3 of the cases) for rotational atherectomy and converting to a solid crown peripheral system in the orbital atherectomy cases occurred frequently, resulting in severe stent disruption of previous integrity and requiring additional stenting in up to 95% of the cases622. Finally, treatment using contrast-enhanced excimer laser is not widely available; it requires specific training to safely deliver and requires precise “in-stent” applications prohibiting its use in underexpanded stent edges23.
In contrast, the IVL system consists of a balloon dilatation catheter that uses the rapid-exchange method over a regular PCI guidewire to facilitate preparation, delivery and deployment, avoiding the need for extensive operator training or device preparation. Moreover, by transforming acoustic waves into mechanical energy, IVL therapy is capable of reaching the totality of the vessel circumference, thereby effectively disrupting superficial and deeply embedded calcium depositions8. The latter was reflected in our study by a significant gain in both lumen and stent areas, despite the presence of neointimal hyperplasia and/or multiple stent layers in some cases (Central illustration, Figure 2).
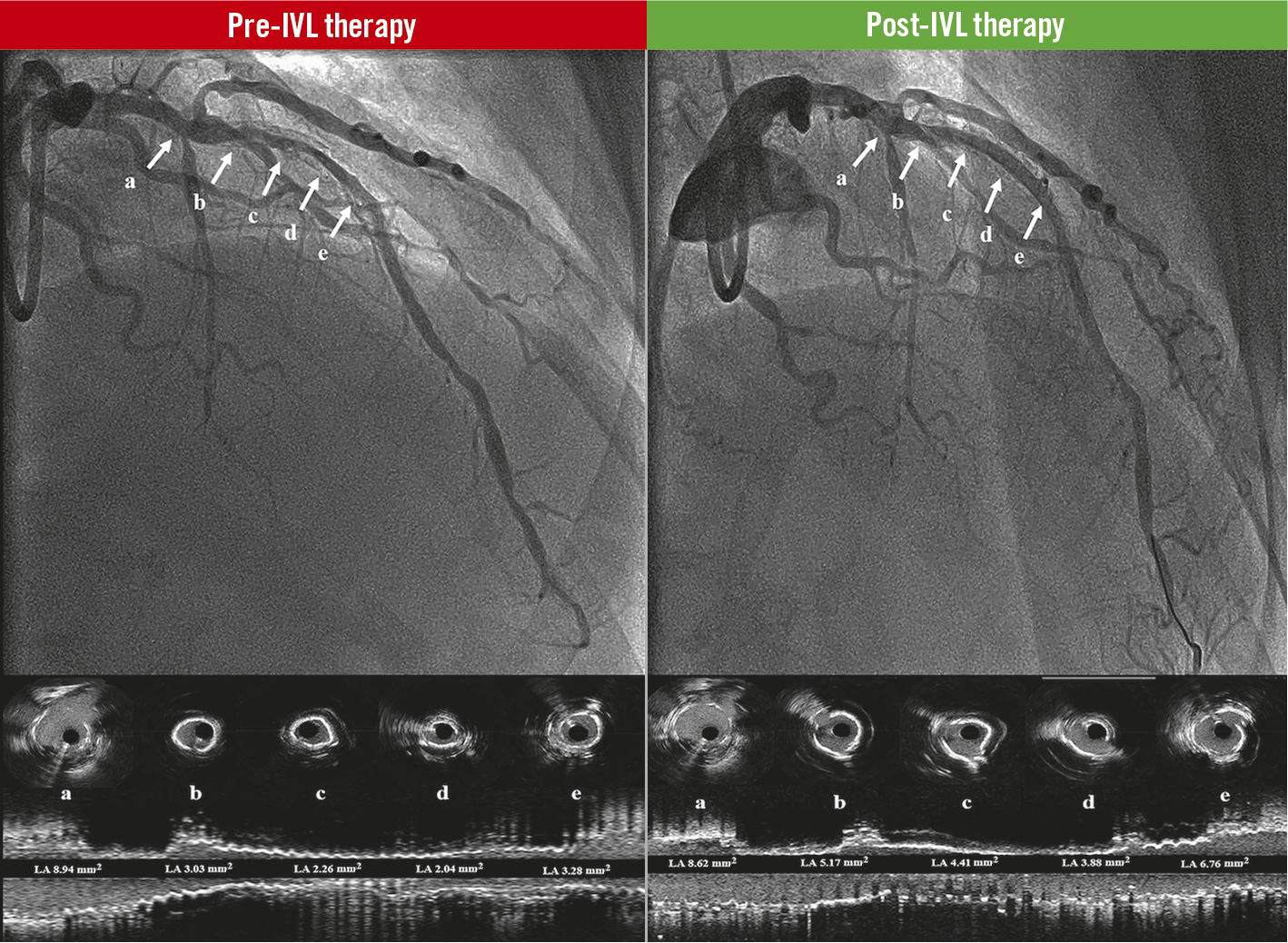
Central illustration. Coronary lithotripsy effect in stent underexpansion. Pre-IVL therapy: baseline angiogram of the LAD and the corresponding coronary intravascular ultrasound cross sections (a, b, c, d, e), and longitudinal view of the vessel. The white arrows show significant stent underexpansion with lumen obstruction. Post-IVL therapy: final result angiograms after coronary lithotripsy therapy of the LAD and the corresponding coronary intravascular ultrasound cross sections (a, b, c, d, e), and longitudinal view of the vessel. The white arrows show significant improvement of stent expansion and lumen obstruction. IVL: intravascular coronary lithotripsy; LAD: left anterior descending coronary artery
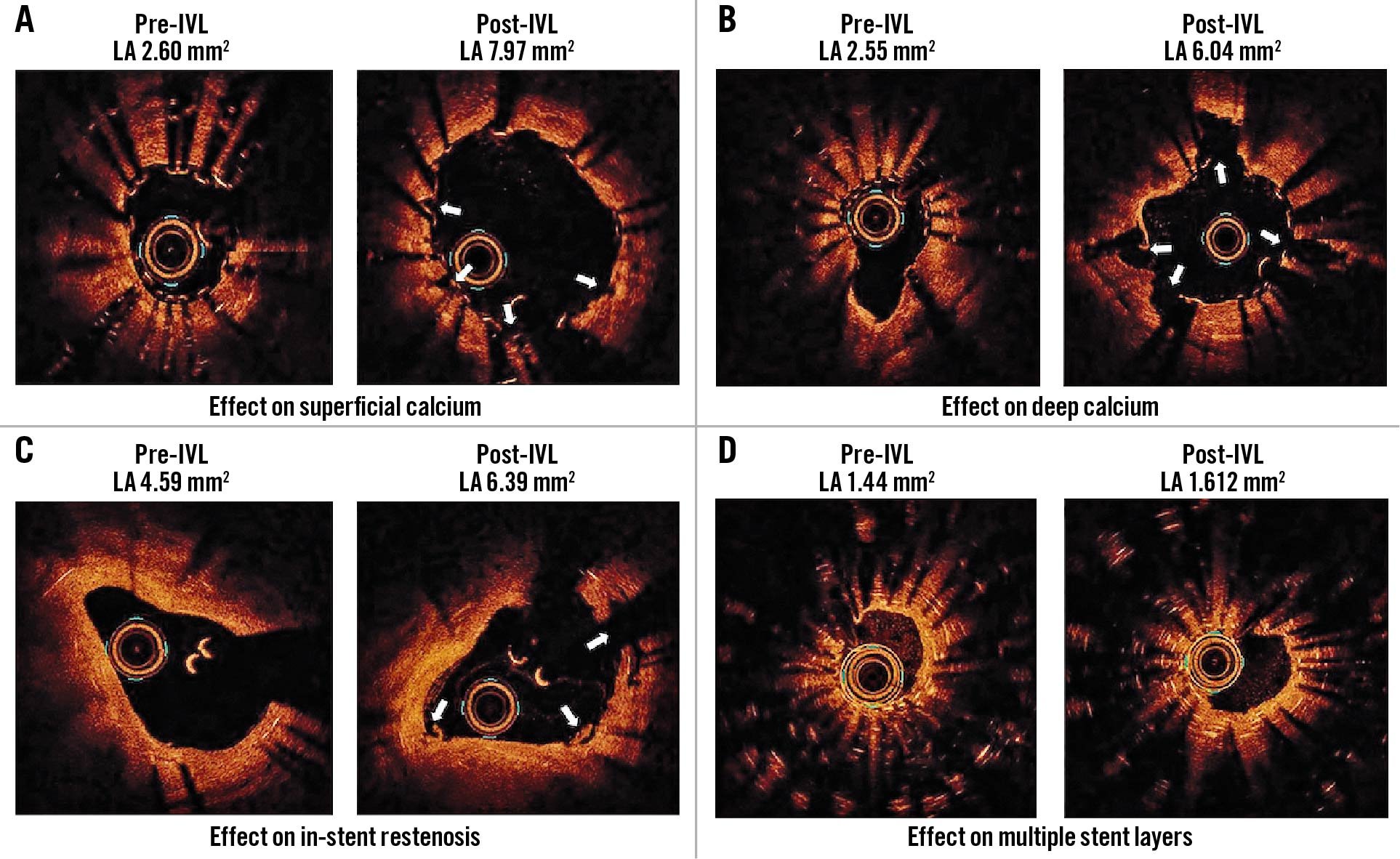
Figure 2. Effect of coronary lithotripsy. Optical coherence tomography transversal views of stented segments. A. Superficial calcium. B. Deep calcium. C. In-stent restenosis. D. Multiple stent layers. IVL: intravascular lithotripsy
A recent study on the efficacy of the IVL therapy for the treatment of SU (n=34) included >97% of old, underexpanded stents (median 13 months, interquartile range 2.5-29 months) and showed device success in only 87.1% of the cases, with a lower IVL efficacy in multilayer stents (5.1% of the cases included)24. In contrast, our registry (n=70) presents a wide range of potential scenarios for IVL in stent underexpansion (from freshly implanted [41.4%] to very old stents [more than 5 years]) and exhibits a higher rate of device success (92.3%), with similar efficacy of the IVL therapy in the presence of multiple stent layers (21.4% of the cases included). Of note, the SMILE registry based their definition of device success on QCA and/or intracoronary imaging data reported by each participating site. In contrast, our data were analysed by a central core lab, reducing potential biases in the analyses.
An exploratory analysis, aimed at finding predictors of IVL failure to achieve lumen diameter gain, found that both ostial location of the target stented segment and the use of IVL therapy as a bailout strategy had a negative predictive effect. Of note, the presence of multiple layers of stents was not associated with IVL failure. Ostial atherosclerotic tissue tends to be thicker with a higher content of fibro-cellular and sclerotic fragments, making it more resistant to dilatation and prone to recoil25. This adds complexity to an already established technical difficulty increasing the likelihood of target lesion failure. On the other hand, we found that IVL therapy seemed less effective in newly implanted stents. Increased awareness of the impact of coronary calcification among the interventional community over the last years has certainly translated into more aggressive periprocedural attempts to correct stent underexpansion at the time of the index PCI procedure. Suboptimal results occurred only in those cases with more unfavourable features that may also affect IVL performance26. Lesion interrogation by invasive imaging may arguably enhance the operator’s insight into the calcium burden of a target lesion and help define the optimal treatment strategy, including plaque modification, to prevent subsequent SU prior to stent implantation (Central illustration).
In the CRUNCH registry, IVL therapy was used as a bailout strategy directly after DES implantation in 43.3% of the cases. Hypothetically, IVL could have a substantial effect on the stent platform and antiproliferative coating, causing cracks or detachments that may affect the biological performance of the DES in facilitating vessel healing. For this reason, the use of IVL in this context remains a bailout procedure. Extensive lesion preparation ensuring proper calcium modification should be fundamental before considering stent implantation, and stenting should be avoided in cases with unsatisfactory results. The nature of the present in vivo study prevented us from making any statements on the potential negative impact of IVL therapy on stent backbone/polymer; however, a recent case report confirmed stent patency and integrity at 4-month follow-up, with angiography and OCT assessment27.
The treatment of SU with debulking devices entails high rates of procedural complications and adverse clinical events. Of note, 15.4%, 5% and 3.6% of periprocedural myocardial infarction (MI) has been described with RA, OA and contrast-enhanced excimer laser, respectively62021. Considering that ablative techniques rely on direct contact with the vessel wall to exert thermal and mechanical plaque modification, there is a higher rate of coronary dissection, perforation, flow impairment, stent disruption, distal embolisation of microparticles and device entrapment62829. Conversely, the IVL system does not rely on barotrauma to enhance vessel compliance; therefore, a lower incidence of coronary flow disturbance is expected1314151617. A recently published pooled data analysis (n=628), from all the DISRUPT CAD studies, supports the lower rate of adverse events (up to 6.8%), mainly driven by periprocedural MI12. This is in line with the results of an all-comer registry (n=71), where no IVL-related complications or MACE were reported30.
Although the use of IVL for the treatment of stent underexpansion remains “off-label”, our study highlights that IVL therapy is an effective and safe strategy, especially when other devices have failed or entail an unacceptable, higher risk for complications. Further data on its short- and long-term safety are warranted.
Limitations
The present study was subject to several limitations of note. First, given the observational character of the present study, the selection of patients and target lesions, the timing of IVL use, the use of other plaque modification devices, and additional stenting were left to the operator’s discretion. As a consequence, due to the adjuvant character of the IVL therapy in the present study, along with the non-protocolised use of intravascular imaging and the difficulty in identifying struts in the proximity of calcifications in the IVUS pullbacks, we were not able to completely disentangle the sole local effect of the IVL therapy. Second, angiographic and intracoronary imaging data suitable for paired analysis were not available in all cases. Third, no systematic collection of cardiac biomarkers was mandated post-procedure. Therefore, the incidence of periprocedural MI cannot be accurately ruled out. Fourth, no long-term follow-up was available. Hence, our results are hypothesis-generating and require confirmation by larger-scale randomised studies evaluating the efficacy and safety of IVL therapy versus other available devices in this specific context, and its impact on the stent backbone/polymer and drug elution.
Conclusions
Coronary lithotripsy is a safe and effective strategy to treat persistent stent underexpansion secondary to heavily calcified lesions.
Impact on daily practice
There are limited options to tackle persistent stent underexpansion due to undilatable calcific lesions, besides non-compliant balloon dilatations at high and super-high pressure. Our results show that intravascular lithotripsy therapy is safe and effective in improving stent underexpansion in this context.
Conflict of interest statement
G. Sardella received speaker's fees from Shockwave Medical. N. Werner received grant support/speaker honorarium from Abiomed, AstraZeneca, Abbott Vascular, Boston Scientific, ACIST Medical, Daiichi Sankyo, Medtronic, Edwards Lifesciences, and Shockwave Medical. J. Escaned reports personal fees as a speaker or consultant from Abbott, Boston Scientific, Medtronic, Shockwave Medical, and Philips. F. Ugo acted as a proctor last year and presently for LAA closure for Boston Scientific, acts as a proctor presently for LAA closure for Gada/Lifetech, and received a speaker’s fee for a webinar for Terumo Europe. N. M. Van Mieghem has received institutional grants from Abbott Vascular, Boston Scientific, Medtronic, PulseCath BV, Daiichi Sankyo, and Abiomed. J. Daemen received institutional grant/research support from AstraZeneca, Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic, Pie Medical, and ReCor Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.




