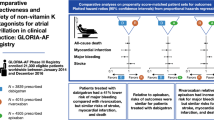
Abstract
Background
Prospectively collected, routine clinical practice-based data on antithrombotic therapy in non-valvular atrial fibrillation (AF) patients are important for assessing real-world comparative outcomes. The objective was to compare the safety and effectiveness of dabigatran versus vitamin K antagonists (VKAs) in patients with newly diagnosed AF.
Methods and results
GLORIA-AF is a large, prospective, global registry program. Consecutive patients with newly diagnosed AF and CHA2DS2-VASc scores ≥ 1 were included and followed for 3 years. To control for differences in patient characteristics, the comparative analysis for dabigatran versus VKA was performed on a propensity score (PS)-matched patient set. Missing data were multiply imputed. Proportional-hazards regression was used to estimate hazard ratios (HRs) for outcomes of interest. Between 2014 and 2016, 21,300 eligible patients were included worldwide: 3839 patients were prescribed dabigatran and 4836 VKA with a median age of 71.0 and 72.0 years, respectively; > 85% in each group had a CHA2DS2-VASc-score ≥ 2. The PS-matched comparative analysis for dabigatran and VKA included on average 3326 pairs of matched initiators. For dabigatran versus VKAs, adjusted HRs (95% confidence intervals) were: stroke 0.89 (0.59–1.34), major bleeding 0.61 (0.42–0.88), all-cause death 0.78 (0.63–0.97), and myocardial infarction 0.89 (0.53–1.48). Further analyses stratified by PS and region provided similar results.
Conclusions
Dabigatran was associated with a 39% reduced risk of major bleeding and 22% reduced risk for all-cause death compared with VKA. Stroke and myocardial infarction risks were similar, confirming a more favorable benefit-risk profile for dabigatran compared with VKA in clinical practice.
Clinical trial registration https://www.clinicaltrials.gov. NCT01468701, NCT01671007.
Graphical abstract

Similar content being viewed by others
Introduction
Thromboembolic complications are a major cause of morbidity and mortality in patients with non-valvular atrial fibrillation (AF), and while anticoagulation reduces the risk of ischemic stroke, the risk of bleeding is an impediment to broad and sustained implementation [1]. The introduction of the non-vitamin K antagonist oral anticoagulants (NOACs) has profoundly changed anticoagulation management for patients with AF, because NOACs offer greater convenience and net clinical benefit compared with vitamin K antagonists (VKAs) [2]. Four pivotal clinical trials have shown NOACs to be at least non-inferior in the prevention of stroke or systemic embolism in patients with AF and to reduce the risk of intracranial hemorrhage compared with VKA [3,4,5,6].
High-quality, practice-based evidence can provide important supplementary data by including patients with characteristics that are under-represented in clinical trials [7]. Retrospective analyses support the safety and effectiveness of dabigatran compared with VKAs or other NOACs in practice [8,9,10] and a large cohort of Medicare patients exhibited a lower risk of ischemic stroke and intracranial hemorrhage when treated with dabigatran compared with warfarin [10, 11]. To date, prospectively collected observational data are less common.
GLORIA-AF (Global Registry on Long-Term Oral Antithrombotic Treatment in Patients With Atrial Fibrillation) [12] is one of the first large, global, prospective registry programs to provide comparative outcome data for dabigatran versus VKA in routine practice [12, 13]. We describe herein the antithrombotic treatment for stroke prevention in Phase III of GLORIA-AF and report the comparative outcomes of dabigatran versus VKA from the final 3-year follow-up, which was the main objective of GLORIA-AF Phase III.
Methods
Study design and setting
GLORIA-AF was an international, multi-center, non-interventional registry program, based on prospectively collected data for patients with newly diagnosed AF. Participating centers were selected to achieve a country-specific balance of health care settings. The three-phased design of the GLORIA-AF Registry Program has previously been published (Fig. 1) [12]. To reduce confounding, Phase III of the program only started once relevant baseline characteristics of patients initiating dabigatran and VKA in Phase II were sufficiently similar to allow for comprehensive comparative analysis, as determined by propensity score (PS) methodology. All patients in GLORIA-AF were managed according to local clinical practice and treatment decisions were solely at the discretion of the treating physician. Patients included in Phase III were followed for 3 years, regardless of prescribed antithrombotic therapy.
GLORIA-AF was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki and Good Epidemiological Practice and Good Pharmacoepidemiology Practices, and the protocol was approved by the European Medicines Agency and institutional review boards at each participating site. Patients provided written informed consent. An independent, academic steering committee oversaw the design, execution, and study conduct, and was responsible for manuscript development. Extensive measures were undertaken to ensure accurate and complete reporting of outcomes and minimize loss to follow-up. Clinical data and site characteristics were captured using a web-based system over a secure network to ensure confidentiality and data integrity. The Data Sharing Statement is included in the Supplementary Information.
Patients
Physicians were encouraged to enroll consecutive patients who met the inclusion criteria. Eligible patients had a recent diagnosis of AF (< 3 months; except in Latin America, where < 4.5 months was used due to referral patterns), were aged ≥ 18 years, had a risk of stroke (CHA2DS2-VASc score ≥ 1), and provided written informed consent. Detailed inclusion and exclusion criteria are in Methods 1 in the Supplementary Information.
Clinical outcomes
Key outcomes (without ranking) were stroke (hemorrhagic, ischemic, and uncertain classification); major bleeding (defined using International Society on Thrombosis and Haemostasis criteria); myocardial infarction; all-cause death; life-threatening bleeding; and the composite of stroke, systemic embolism, myocardial infarction, vascular death, and life-threatening bleeding (definitions in Methods 2 in the Supplementary Information).
Statistical methods
The Statistical and Epidemiological Analysis Plan was finalized before database lock. Demographics and baseline characteristics were summarized descriptively. Baseline characteristics were compared between dabigatran and VKA patients within different patient sets in terms of standardized differences. For outcome analyses regarding dabigatran and VKAs, missing data for baseline covariates and cause of death were handled using multiple imputation (Methods 3 in the Supplementary Information). All outcome analyses were performed separately for each imputed patient set, and results combined to provide estimates under the missing-at-random assumption. Analyses were performed using SAS® software version 9.4 or later (SAS Institute, Inc., Cary, NC).
Patient sets Per protocol, two patient sets were predefined to adjust for unbalanced baseline characteristics in the dabigatran and VKA patient arms.
The PS-trimmed set consisted of a subset of patients obtained after excluding those in the non-overlapping tails of the PS distribution (PS trimming) within each geographic region (Methods 4 in the Supplementary Information). Excluding these patients from the tails of the PS distribution addresses channeling bias and improves the validity of comparisons. The PS-matched set was generated from the PS-trimmed patient set by 1:1 greedy nearest-neighbor matching of dabigatran patients to VKA patients, with a predefined caliper, within region (Methods 4 in the Supplementary Information). Descriptive analyses for the PS-trimmed and PS-matched sets are based on the PS calculated using the first of the multiple imputation patient sets, i.e., the first trimmed and matched sets.
Clinical outcome analyses Incidence rates with 95% confidence intervals (CIs) of the key outcome events were calculated for all treatment groups within the eligible patients (Supplementary Table S1). For dabigatran and VKA, incidence rates with 95% CI of the key outcomes were additionally calculated in the PS-trimmed and PS-matched patient sets. The initial analysis comparing effects of dabigatran with VKA was conducted using a multivariable Cox regression model within the PS-trimmed patient set. The model included treatment, age, sex, and risk factors for stroke and bleeding as core variables in the model. Further variables were included based on covariate selection procedures (Methods 5 in the Supplementary Information). Hazard ratios (HRs) with 95% CIs were presented for outcomes considered. The comparative analyses were also conducted in the PS-matched patient set by Cox regression with a shared frailty factor to adjust the matching [14]. Among the matched patients, the degree of balance between dabigatran and VKA for the individual, prespecified covariates (Supplementary Table S2) was assessed. Any covariate with a standardized difference > 10% was considered unbalanced and was also included as a separate variable in the final proportional-hazards regression model. Kaplan–Meier curves were plotted based on the matched patients for a graphical comparison (Methods 6 in the Supplementary Information). Additionally, we conducted a PS stratification analysis, based upon strata formed by deciles of an extended PS and geographic region (Methods 6 in the Supplementary Information).
Longitudinal outcomes were based on an as-treated approach, censoring patients after permanent discontinuation of initial treatment or study termination (definition of permanent discontinuation in Methods 2 in the Supplementary Information).
Results
Study patients and baseline characteristics
Between January 2014 and December 2016, 21,591 patients were enrolled at 935 sites from 38 countries, of whom 21,300 were eligible for analysis. Their baseline characteristics are in Supplementary Table S3. Approximately 48% were from Europe, while 24%, 20%, and 8% were from North America, Asia, and Latin America, respectively (Supplementary Fig. S1). Of the 21,300 eligible patients, a total of 17,140 (80.5%) patients completed the planned 3 years of observation time. If a patient did not complete the planned observation time, vital status information was collected where possible. At the end of the study, vital status was available for all but 997 (4.7%) eligible patients who did not complete the planned observation time and had no information on vital status available (i.e., alive or dead).
The 20,860 “treated” patient population comprised eligible patients who were prescribed an antithrombotic agent and received at least 1 dose of the treatment (19,718 patients) and patients not prescribed an antithrombotic who had no antithrombotic started at baseline (1142 patients). Of the 20,860 patients, 12,577 patients (60.3%) received a NOAC, 4788 (23.0%) VKA, 2140 (10.3%) acetylsalicylic acid alone, 213 (1.02%) antiplatelet other than acetylsalicylic acid, and 1142 (5.5%) had no antithrombotic treatment (Fig. 2).
Table 1 shows baseline characteristics of the eligible patients treated with dabigatran (n = 3839) or VKA (n = 4836).
Patient flow. Data are from the patient set determined by the first of the 20 imputed datasets. ASA acetylsalicylic acid; OAC oral anticoagulant; PS propensity score; VKA vitamin K antagonist. aEligible patient set includes patients who were prescribed but did not take the antithrombotic therapies. This includes dabigatran (n = 32) and VKA (n = 52). These patients are excluded from the subsequent outcome analyses. bNs from individual treatment groups do not add up to the total treated N as we do not show all treatments and treatment combinations. cIn the dabigatran and VKA groups, patients with a PS less than the 1.5th percentile of the PS distribution for the dabigatran-exposed group and those with PS larger than the 98.5th percentile of the PS distribution for the VKA-exposed group were excluded. dLoss to follow-up is defined as not completed planned observation time and no information on vital status available
Baseline characteristics and incidence rates of PS-trimmed patients
In the PS-trimmed patient set, there were 3609 patients treated with dabigatran and 4421 treated with VKAs. Their baseline characteristics are in Supplementary Table S4. In the dabigatran group, 52.6% received 150 mg twice daily (BID), 45.1% received 110 mg BID, and 1.4% 75 mg BID. A total of 3040 (84.2%) dabigatran and 3538 (80.0%) VKA patients completed the 3-year follow-up. The mean (SD) duration of exposure was 25.2 (14.3) months for dabigatran and 24.3 (14.2) months for VKA. The incidence rates for dabigatran and VKA for stroke, major bleeding, and all-cause death are in Table 2.
Baseline characteristics and incidence rates of the PS-matched patients
In the PS-matched patient set, 3327 patients were included in each of the 2 treatment groups; their baseline characteristics are shown in Supplementary Table S4.
Specifically, the PS-matched patient set led to the dabigatran and VKA cohorts being closely balanced for all covariates. Table 2 shows incidence rates for dabigatran and VKA in the PS-matched patient set, while the incidence rates from the eligible patient population (before multiple imputation) are in Supplementary Table S5.
Comparative analysis of the PS-trimmed and PS-matched patients
Cox regression analysis within the PS-trimmed patient set (Fig. 3A) shows that patients treated with dabigatran had reduced risk for major bleeding (HR 0.52; 95% CI 0.38–0.73), all-cause death (HR 0.66; 95% CI 0.54–0.80), and the composite of stroke, systemic embolism, myocardial infarction, vascular death, and life-threatening bleeding (HR 0.74; 95% CI 0.60–0.90) compared with VKAs. Stroke and myocardial infarction risks were similar (HR 0.81; 95% CI 0.57–1.14 and HR 0.97; 95% CI 0.60–1.57, respectively).
Comparison of outcomes in patients treated with dabigatran or VKA at year 3: (a) in the PS-trimmed patient set (primary analysis); (b) in the PS-matched patient set with adjustment for unbalanced variables. CI confidence interval; CrCl creatinine clearance; HR hazard ratio; PS propensity score; VKA vitamin K antagonist. aAs the PS was calculated using baseline covariates with missing baseline covariates handled by multiple imputation, every patient had 20 estimated PSs, so there were 20 different PS-trimmed patient sets. Results presented are based on the average of the results from those sets. bCensoring patients after permanent discontinuation of initial treatment or study termination. cMultivariable Cox regression models were used to analyze comparative outcomes of dabigatran versus VKAs, along with a covariate selection procedure (see statistical methods section). dComposite outcome: stroke, systemic embolism, myocardial infarction, vascular death, and life-threatening bleeding, eTreatment, along with unbalanced parameters, are considered in the Cox regression model with a shared frailty factor. CrCl, previous oral anticoagulant use, and type of atrial fibrillation were adjusted in the model, as their standardized difference was > 10% in the matched datasets
Outcomes for dabigatran versus VKA within the PS-matched set adjusting for unbalanced variables in the Cox model (Fig. 3B) showed that patients treated with dabigatran had reduced risk of major bleeding (HR 0.61; 95% CI 0.42–0.88) and all-cause death (HR 0.78; 95% CI 0.63–0.97) compared with VKA. HR was 0.85 (95% CI 0.67–1.07) for the composite outcome.
Similar risks for stroke and myocardial infarction were observed, which is consistent with the results from the Cox regression analysis within the PS-trimmed set. Figure 4 shows Kaplan–Meier curves of outcomes in the PS-matched set.
Further sensitivity analyses with stratification using a post hoc PS extended sensitivity analysis (Supplementary Fig. S2) showed that the pattern of results was again consistent with the above-described prespecified analyses.
Discussion
This is the largest prospective, global cohort of consecutive dabigatran- and VKA-treated patients to report long-term follow-up of routine practice-based data comparing the safety and effectiveness of dabigatran with VKAs in patients newly diagnosed with AF. Our principal findings are that, when compared with VKA patients over 3 years of follow-up, dabigatran-treated patients were at reduced risk for major bleeding and all-cause death, with a similar risk of stroke and myocardial infarction. To date, there are only assessments of clinical trial and retrospective analyses comparing dabigatran with VKAs, but GLORIA-AF now provides high-quality data from a prospective global registry.
Prospectively collected, routine, clinical-practice-based data on antithrombotic therapy in non-valvular AF patients are important for assessing real-world comparative outcomes. One specific challenge of comparative analyses in observational studies is differences in patient characteristics based on prescribing information for the drugs of interest as well as physicians’ prescribing preferences that could introduce bias. The design of GLORIA-AF helped to limit bias because Phase III of the study only started in a specific region once relevant baseline characteristics of patients initiating dabigatran and VKA therapy in Phase II showed substantial overlap in the PS distributions [12].
We addressed some of the observed differential prescribing patterns of dabigatran and VKAs using two defined patient sets (PS-trimmed and PS-matched), leading to a better balance for important baseline characteristics. The main drawback of matching is discarding some of the available data; to address this our other analyses, using methods that retain all the patients in the trimmed dataset, preserved precision with otherwise similar findings.
The GLORIA-AF results can be set in context with those using retrospective claims databases to compare dabigatran versus warfarin in various populations. A study of 134,414 elderly US Medicare AF patients treated with dabigatran versus warfarin found lower risk of ischemic stroke (HR 0.80; 95% CI 0.67–0.96), intracranial hemorrhage (HR 0.34; 95% CI 0.26–0.46), and death (HR 0.86; 95% CI 0.77–0.96). Risk of major bleeding was similar (HR 0.97; 95% CI 0.88–1.07) [10]. An analysis of US Department of Defense claims [8] also found risk of stroke (HR 0.73; 95% CI 0.55–0.97), intracranial bleeding (HR 0.49; 95% CI 0.30–0.79), and death (HR 0.64; 95% CI 0.55–0.74) associated with dabigatran treatment were lower compared with warfarin treatment, while major bleeding (HR 0.87; 95% CI 0.74–1.03) was similar. It needs to be considered that these US-based analyses mainly include dabigatran 150 mg BID and a small minority of 75 mg BID, as dabigatran 110 mg BID is not approved in USA. This might also explain the better safety of dabigatran that was observed in GLORIA-AF, in which 45% of dabigatran patients received dabigatran 110 mg BID, a dose that physicians can select for patients at risk of bleeding.
Comparable results, albeit with shorter mean duration of follow-up compared with GLORIA-AF, have also been reported from Danish prescription and patient registries (13 months) [9]. The Danish registry presented data separately for different dabigatran doses. Risk for major bleeding in the VKA-naïve patients was lower versus VKAs for dabigatran 150 mg BID (HR 0.67; 95% CI 0.53–0.85) and similar for dabigatran 110 mg BID (HR 0.91; 95% CI 0.73–1.14); intracranial bleeding risk was lower with both doses of dabigatran versus VKAs (HR 0.32; 95% CI 0.16–0.63 for 150 mg BID and HR 0.31; 95% CI 0.17–0.55 for 110 mg BID) [9].
Two other large registries assessing treatment patterns from routine clinical practice and outcomes in newly diagnosed patients with AF are the Global Anticoagulant Registry in the Field (GARFIELD)-AF and Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF) [15, 16]. In the GARFIELD-AF registry, major bleeding and all-cause mortality were lower with NOACs than VKAs (0.79 [0.70–0.89]; 0.77 [0.61–0.98]), respectively. However, GARFIELD-AF was not a global registry, as North American patients were not included and follow-up was limited to 2 years [16]. Therefore, GLORIA-AF is the only global study providing long-term comparative data complementing the outcomes of pivotal trials through the use of unselected real-world populations and conditions.
GLORIA-AF highlights the value of routine practice data where the clinical outcomes are based on the actual use of dabigatran, including dose selection. The rates of clinical outcomes in this population from the GLORIA-AF study were low and showed a favorable benefit-risk profile for dabigatran compared with VKAs. When comparing the results of GLORIA-AF with the pivotal Phase III trial RE-LY, where patients were randomly assigned to receive either dabigatran 150 mg BID, dabigatran 110 mg BID, or VKAs, dabigatran patients compared with VKA patients in RE-LY had similar or lower rates of major bleeding, while patients taking dabigatran 150 mg BID had lower ischemic stroke rates [6, 17].
The differences between GLORIA-AF and RE-LY are likely due to different study designs such as the randomization of dabigatran doses and differences in the patient populations. For example, GLORIA-AF included only newly diagnosed AF patients, whereas in RE-LY, two-thirds of the patients had pre-existing AF. Furthermore, in GLORIA-AF, the mean CHADS2 and HAS-BLED scores were 1.8 and 1.2, compared with mean scores of 2.1 and 1.3, respectively, in RE-LY. Regarding concomitant diseases, while median age, history of hypertension, and diabetes mellitus were similar, history of heart failure was 32% in RE-LY and 21% in GLORIA-AF. Prior stroke/transient ischemic attack history was 20% in RE-LY, while prior stroke/transient ischemic attack/systemic embolism was 14% in GLORIA-AF.
In the RE-LY trial, numerically more myocardial infarction events were observed with dabigatran compared with VKAs [18]. A meta-analysis including > 300,000 patients from retrospective analyses, indicated that the risk for myocardial infarction was similar between dabigatran- and VKA-treated patients [19]. These data are now complemented by the prospective data from GLORIA-AF, in which a similar myocardial infarction risk for dabigatran compared with VKA was also observed. Importantly, follow-up for the GLORIA-AF cohorts was 3 years, the longest described so far in a comparative assessment.
Limitations and strengths
The generalizability of our study results may be limited by the fact that the study population was restricted to those with a CHA2DS2-VASc score ≥ 1, though this is similar to other contemporary AF registries. Furthermore, nearly 50% of the cohort was enrolled in Europe. Sites included in GLORIA-AF had to have access to and be able to prescribe both dabigatran and VKAs. Consequently, treatment patterns could be influenced by site selection. To minimize selection bias at the patient level, physicians were encouraged to enroll consecutive consenting patients who met the inclusion criteria.
With no randomization, the study may be subject to confounding by factors not adjusted for in the analysis. Cause of death was unknown in approximately 20% of deaths; for these patients, multiple imputation was used for comparative analysis. Thus, bias may be introduced if the assumptions behind the imputation procedure were not held. To assess the potential impact of this procedure, a sensitivity analysis was performed where unknown death was imputed as vascular cause and a second where unknown death was imputed by non-vascular cause (data on file). The data obtained by these sensitivity analyses were in line with the results observed in the primary analysis.
Strengths of the GLORIA-AF study include the fact that it is the largest prospective global cohort of consecutive dabigatran- and VKA-treated patients reported thus far. Over the 3-year observation period, regular follow-up with physicians, alongside 10% on-site monitoring, and multiple standards for data quality assurance and review ensured event capture. As such, the data quality is strong for an observational setting, with low percentage of eligible patients for whom no information on vital status was available (4.7%). The prospective study design complements other published studies based on retrospective data sources.
Conclusions
In conclusion, this is the largest, global, prospective cohort of consecutive dabigatran and VKA patients, to report clinical practice-based data comparing the safety and effectiveness of dabigatran with VKAs for stroke prevention in a broad AF patient population. After 3 years' follow-up, the risks of major bleeding and all-cause death for dabigatran were lower compared with VKAs, while the risks for stroke and myocardial infarction were similar. These results confirm a more favorable benefit-risk profile for dabigatran compared with VKAs in routine clinical practice and add to the evidence available for dabigatran in newly diagnosed AF patients, by complementing results from the pivotal RE-LY trial and retrospective analyses performed on various patient populations.
Data availability
Please see Data Sharing Statement in the Supplementary Information.
Code availability
Not applicable.
References
Hindricks G, Potpara T, Dagres N et al (2020) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa612
Lip G, Freedman B, De Caterina R, Potpara TS (2017) Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost 117(7):1230–1239. https://doi.org/10.1160/th16-11-0876
Patel MR, Mahaffey KW, Garg J et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891. https://doi.org/10.1056/NEJMoa1009638
Granger CB, Alexander JH, McMurray JJ et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365(11):981–992. https://doi.org/10.1056/NEJMoa1107039
Giugliano RP, Ruff CT, Braunwald E et al (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369(22):2093–2104. https://doi.org/10.1056/NEJMoa1310907
Connolly SJ, Ezekowitz MD, Yusuf S et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361(12):1139–1151. https://doi.org/10.1056/NEJMoa0905561
Franklin JM, Glynn RJ, Martin D, Schneeweiss S (2019) Evaluating the use of nonrandomized real-world data analyses for regulatory decision making. Clin Pharmacol Ther 105(4):867–877. https://doi.org/10.1002/cpt.1351
Villines TC, Schnee J, Fraeman K et al (2015) A comparison of the safety and effectiveness of dabigatran and warfarin in non-valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost 114(6):1290–1298. https://doi.org/10.1160/th15-06-0453
Larsen TB, Gorst-Rasmussen A, Rasmussen LH, Skjoth F, Rosenzweig M, Lip GY (2014) Bleeding events among new starters and switchers to dabigatran compared with warfarin in atrial fibrillation. Am J Med 127(7):650-656.e655. https://doi.org/10.1016/j.amjmed.2014.01.031
Graham DJ, Reichman ME, Wernecke M et al (2015) Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 131(2):157–164. https://doi.org/10.1161/circulationaha.114.012061
Graham DJ, Baro E, Zhang R et al (2019) Comparative stroke, bleeding, and mortality risks in older Medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med 132(5):596-604.e511. https://doi.org/10.1016/j.amjmed.2018.12.023
Huisman MV, Lip GY, Diener HC et al (2014) Design and rationale of Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation Am Heart J 167(3):329–334. https://doi.org/10.1016/j.ahj.2013.12.006
Mazurek M, Teutsch C, Diener HC et al (2019) Safety and effectiveness of dabigatran at 2 years: final outcomes from phase II of the GLORIA-AF registry program. Am Heart J 218:123–127. https://doi.org/10.1016/j.ahj.2019.08.012
Duchateau L, Janssen P (2008) The frailty model. Springer
Jackson LR 2nd, Kim S, Fonarow GC et al (2018) Stroke risk and treatment in patients with atrial fibrillation and low CHA2DS2-VASc scores: findings from the ORBIT-AF I and II registries. J Am Heart Assoc 7(16):e008764. https://doi.org/10.1161/JAHA.118.008764
Camm AJ, Fox KAA, Virdone S et al (2021) Comparative effectiveness of oral anticoagulants in everyday practice. Heart. https://doi.org/10.1136/heartjnl-2020-318420
Connolly SJ, Wallentin L, Yusuf S (2014) Additional events in the RE-LY trial. N Engl J Med 371(15):1464–1465. https://doi.org/10.1056/NEJMc1407908
Hohnloser SH, Oldgren J, Yang S et al (2012) Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 125(5):669–676. https://doi.org/10.1161/CIRCULATIONAHA.111.055970
Ntaios G, Papavasileiou V, Makaritsis K, Vemmos K, Michel P, Lip GYH (2017) Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke 48(9):2494–2503. https://doi.org/10.1161/STROKEAHA.117.017549
Acknowledgements
Medical writing support was provided by Keith Day, PhD, of Parexel, with funding from Boehringer Ingelheim. Dr Miney Paquette and Laurie Baker are acknowledged for supporting the study conduct for many years. For programming and data management, Ralf Minkenberg, Paul Allison, Pol Mac-an-Mhaoir, and Holger Jaumann are acknowledged. The authors thank the patients who participated in this trial, their families, the investigators, study coordinators, study teams, and nurses.
Funding
This study was supported by Boehringer Ingelheim.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
MVH reports grants from ZonMW Dutch Healthcare Fund, and grants and consultation honoraria from Boehringer Ingelheim, Pfizer/Bristol-Myers Squibb, Bayer Health Care, Aspen, and Daiichi-Sankyo. H-CD reports honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, Portola, and WebMD Global. Financial support for research projects was provided by Boehringer Ingelheim. SJD reports consultancy fees for serving as a Steering Committee member for Boehringer Ingelheim and research grants from Abbott (St Jude Medical). JLH reports consulting fees/honoraria or research support from Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo Pharma, Janssen Pharmaceuticals, Johnson & Johnson, Medtronic, Pfizer, and Sanofi Aventis. C-SM reports consultancy fees/honoraria from Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. KJR reports employment by RTI Health Solutions, an independent, non-profit research organization that does work for government agencies and pharmaceutical companies. RL, VKG, and CT report employment by Boehringer Ingelheim International GmbH. DBB reports past employment by Boehringer Ingelheim International GmbH and current employment by UCB pharma. SL reports past employment by Boehringer Ingelheim International GmbH. GYHL reports consultancy for Bayer/Janssen, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi Sankyo; and speaking for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo; no fees are directly received personally.
Ethics approval
GLORIA-AF was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki and Good Epidemiological Practice and Good Pharmacoepidemiology Practices, and the protocol was approved by the European Medicines Agency and institutional review boards at each participating site.
Consent to participate
Patients provided written informed consent.
Additional information
GLORIA-AF investigators are listed in the Supplementary Information.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huisman, M.V., Teutsch, C., Lu, S. et al. Dabigatran versus vitamin K antagonists for atrial fibrillation in clinical practice: final outcomes from Phase III of the GLORIA-AF registry. Clin Res Cardiol 111, 548–559 (2022). https://doi.org/10.1007/s00392-021-01957-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01957-1