Article Text
Abstract
Objective Inflammatory cardiomyopathy is characterised by inflammatory infiltrates leading to cardiac injury, left ventricular (LV) dilatation and reduced LV ejection fraction (LVEF). Several viral pathogens and autoimmune phenomena may cause cardiac inflammation.
The effects of the gain of function FOXO3A single-nucleotide polymorphism (SNP) rs12212067 on inflammation and outcome were studied in a cohort of patients with inflammatory dilated cardiomyopathy (DCMi) in relation to cardiac viral presence.
Methods Distribution of the SNP was determined in virus-positive and virus-negative DCMi patients and in control subjects without myocardial pathology. Baseline and outcome data were compared in 221 virus-negative patients with detection of cardiac inflammation and reduced LVEF according to their carrier status of the SNP.
Results Distribution of SNP rs12212067 did not differ between virus-positive (n=22, 19.3%), virus-negative (n=45, 20.4 %) and control patients (n=18, 23.4 %), indicating the absence of susceptibility for viral infection or inflammation per se (p=0.199). Patients in the virus-negative DCMi group were characterised by reduced LVEF 35.5% (95% CI) 33.5 to 37.4) and increased LVEDD (LV end-diastolic diameter) 59.8 mm (95% CI 58.5 to 61.2). Within the group, SNP and non-SNP carriers had similarly impaired LVEF 39.2% (95% CI 34.3% to 44.0%) vs 34.5% (95% CI 32.4 to 36.5), p=0.083, and increased LVEDD 58.9 mm (95% CI 56.3 to 61.5) vs 60.1 mm (95% CI 58.6 to 61.6), p=0.702, respectively. The number of inflammatory infiltrates was not different in both SNP groups at baseline. Outcome after 6 months showed a significant improvement in LVEF and clinical symptoms in SNP rs12212067 carriers 50.9% (95% CI 45.4 to 56.3) versus non-SNP carriers 41.7% (95% CI 39.2 to 44.2), p≤0.01. The improvement in clinical symptoms and LVEF was associated with a significant reduction in cardiac inflammation (ΔCD45RO+ p≤0.05; ΔMac-1+ p≤0.05; ΔLFA-1+ p≤0.01; ΔCD54+ p≤0.01) in the SNP cohort versus non-SNP cohort, respectively. Subgroup analyses identified ΔMac-1+, ΔLFA-1+, ΔCD3+ and Δperforin+ as predictors for improvement in cardiac function in SNP-positive patients.
Conclusion FOXO3A might act as modulator of the cardiac immune response, diminishing cardiac inflammation and injury in pathogen-negative DCMi.
- myocarditis
- cardiomyopathies
- genetic association studies
- heart failure
- inflammation
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
FOXO3A is a transcription factor involved in immunoregulation.
That has been shown to suppress inflammation in viral inflammatory dilated cardiomyopathy (DCMi) leading to unfavourable outcome.
WHAT THIS STUDY ADDS
Gain of function FOXO3 single-nucleotide polymorphism rs12212067 is associated with attenuated inflammation and improvement of clinical symptoms, as well as remodelling in virus-negative DCMi.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Modulation of FOXO3 activity might be a useful therapeutic target for inflammatory heart disease.
Introduction
With its helix-loop-helix DNA-binding domain, FOXO3A is involved in an abundance of cellular processes, such as cell cycle regulation, apoptosis, oxidative stress, angiogenesis and immunity.1 The activation of FOXO3A is regulated via post-translational modifications. Growth factors lead to phosphorylation/inactivation of FOXO3A via the phospho-inositol-3-kinase (PI3K/Akt) pathway inducing its cytoplasmic sequestration and degradation.2 Oxidative stress or glucose phosphorylate FOXO3A via the AMP-activated protein kinase (AMPK) pathway on specific consensus sites leading to its activation and differential target gene expression. Moreover, sirtuins have been implicated in FOXO3A transcription. Although FOXO3A proteins are expressed by immune cells, their physiological role in immune responses during an infection is still not fully understood. Single nucleotide polymorphisms (SNPs) in the FOXO3A gene are associated with longevity, a better self-rated health and low prevalence of cardiovascular diseases in independent populations.3 Importantly, the human SNP rs12212067 in FOXO3A was recently shown to be associated with an increased risk for pathogen-induced inflammatory disorders but with a milder course in patients with autoimmune disease-induced inflammation.4
The immune-modulatory function of FOXO3A has been elucidated to some extent: studies in Foxo3a-deficient mice showed an association with sustained proliferation and survival of T cells, as well as spontaneous autoimmunity due to increased nuclear factor kappa B (NF-κB) activity, a downstream target of Foxo3a. Foxo3a acts as negative regulator of cell responses in CD8+ T cells5 and decreased expression of Foxo3a stimulates CD8+ T cells to differentiate into cytotoxic CD8+ T cells.6 Cooperatively, Foxo3a and Foxo1 control the differentiation of regulatory T cells via the protein kinase C/Akt pathway and transforming growth factor beta (TGF-β), thereby maintaining immune tolerance. Moreover, Foxo3a prevents naive T cells to acquire T effector functions. In immature B cells, Foxo3a promotes apoptosis and diminishes auto-antibody generation. In macrophages, Foxo3a negatively regulates interleukin-10 (IL-10), which in turn regulates cell polarisation and subsequent adaptive immune responses.
Studies from our group and others have previously shown that Foxo3a plays an important role in cardiac hypertrophy, cardiomyocyte survival, cell differentiation and remodelling. Furthermore, the FOXO3A gain of function SNP rs12212067 diminished the innate immune response resulting in delayed viral clearance, increased cardiac inflammation and attenuated improvement of left ventricular ejection fraction (LVEF) indicating worsened prognosis in mice with coxsackievirus B3-induced myocarditis and patients with virus-positive inflammatory cardiomyopathy (DCMi).7 These effects were at least in part due to modulation of natural killer (NK) cell differentiation and activation, and reduced interferon-γ (IFN-γ) expression leading to an attenuated innate immune response in wild-type mice and SNP carriers.7
Active chronic cardiac inflammation due to post-viral antigen mimicry or cardiac involvement in systemic autoimmune disease is often seen in patients presenting with virus-negative DCMi. In these patients, viral pathogens are not present in endomyocardial specimens characterised by mononuclear inflammatory infiltrates.8 9
We hypothesised that immune modulation by the FOXO3A gain of function SNP rs12212067 is associated with reduced cardiac inflammation and attenuated immune-mediated tissue injury resulting in improved outcomes in this patient cohort.
Materials and methods
Patients
Data of patients with DCMi were retrospectively collected from a tissue and biodata bank of the collaborative research network CRC Transregio 19 (NCT02970227).
Consecutively enrolled patients were included in the study who were older than 18 years, did undergo EMB (endomyocardial biopsies) and TTE (transthoracic echocardiography) at Charité - University Hospital between 2004 and 2010 (see online supplemental file 1 for more information).
Supplemental material
(Immuno-)Histological and molecular diagnostics as well as determination of carrier status
EMBs were obtained in patients after exclusion of coronary artery disease and other possible causes for cardiac dysfunction. Then EMBs were stained and analysed with light and fluorescence microscopy. Immunoreactivity of the inflammatory cells was quantified by digital image analysis. Carrier status was determined by sampling DNA and using genotyping assays based on the principle of allelic discrimination PCR (see online supplemental file 1 for full description). The following criteria defined the virus-negative DCMi group: left ventricular (LVEF) <50%, increased LV end-diastolic diameter (LVEDD) >55 mm and a positive myocardial inflammation score, as well as absence of cardiac viral genomes in the PCR test (see online supplemental file 1).
Statistical analysis
Descriptive statistics included frequencies and percentage or mean values with 95% CI). Based on normality, Mann-Whitney U test or t-test without Bonferroni correction was used. Multivariate linear regression analysis was performed at baseline and T1, adjusted for age, in a subgroup (see online supplemental file 1 for full information). In all cases, a p≤0.05 (with calculated effect size) was regarded as statistically significant. Statistical analyses were performed using SPSS Statistics software V23.0 (IBM, Armonk, New York, USA).
Results
Distribution of FOXO3A SNP rs12212067 in different patient cohorts
FOXO3A SNP rs12212067 presence was determined in patients with virus-negative inflammatory cardiomyopathy (n = 221), virus-positive DCMi (n = 114) and control subjects (n = 77) (online supplemental figure 1). Of note, SNP distribution did not differ in patients with autoimmune (virus-negative) inflammatory cardiomyopathy compared with controls with preserved LVEF and LV dimensions that were negative for intracardiac inflammation (p=0.199). Moreover, the carrier status for FOXO3A SNP rs12212067 was not different from patients with proven virus-positive DCMi. In both DCMi patient cohorts, approximately 20 % of patients were identified as being carriers for the FOXO3A SNP rs12212067, numbers were comparable to control subjects. Moreover, there was no sex-specific distribution in FOXO3A SNP rs12212067 (online supplemental figure 2) in patients with virus-negative inflammatory cardiomyopathy.
Supplemental material
Supplemental material
Patient characteristics at baseline
Out of 221 consecutive patients (age: 50.9 years (95% CI 48.9 to 52.9)) with virus-negative DCMi analysed, 45 were identified as carriers of the FOXO3A SNP rs12212067 (table 1). LVEF was severely reduced with 39.2% (95% CI 34.3% to 44.0%) vs 34.5% (95% CI 32.4% to 36.5%), p=0.083, in the SNP carrier group versus non-SNP carrier group, respectively (figure 1A, figure 2A). Compared with normal subjects (<55 mm), the LVEDD was enlarged with 59.8 mm (95% CI 58.5 to 61.2) in accordance with increased LVEDVI (LV end-diastolic diameter volume index) (figure 1B,C, figure 2B,C, table 1). Baseline characteristics did not significantly differ in carriers versus non-carriers of the SNP. In line with these observations, vital parameters and clinical symptoms did not significantly differ. Analysis for cardiac viral genomes was negative for CoxB3, hepatitis B, enteroviruses and parvovirus B19. Both patient cohorts were characterised by cardiac immune cell infiltration indicating ongoing cardiac inflammation. In accordance with increased expression of immune cell surface or cytoplasmatic markers, immunohistochemical analysis showed increased Mac-1 positive infiltrates without significant differences in cardiac inflammatory marker expression. Comorbidities,10 medication and number of cardiovascular risk factors did not differ among both groups (table 1, online supplemental figures 3 and 4).
Supplemental material
Supplemental material
Baseline characteristics according to genotype of FOXO3A SNP rs12212067 in patients with non-viral cardiomyopathy
Echocardiographic changes of left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD) and left ventricular end-diastolic diameter volume index (LVEDVI) at short-term and long-term follow-up according to FOXO3A single-nucleotide polymorphism (SNP) status. Patients underwent serial echocardiographic examinations for LVEF (A), LVEDD (B) and LVEDVI (C) at baseline, short-term and long-term follow-up. Data are shown as a boxplot. Number of patients characterised is indicated.
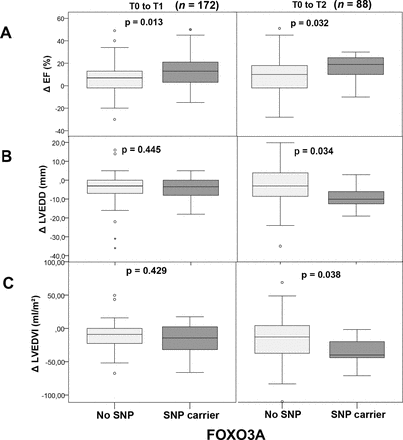
Probability of left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD) and left ventricular end-diastolic diameter volume index (LVEDVI) improvement according to FOXO3A single-nucleotide polymorphism (SNP) status. Patients were classified according to their carrier status. The boxplots show changes in LVEF (ΔLVEF) from baseline (T0) to short-term follow-up (T1) and baseline (T0) to long-term follow-up (T2), respectively, (A) as well as changes in LVEDD (ΔLVEDD) (B) and LVEDVI (ΔLVEDV Index) (C) from baseline (T0) to short-term (T1) and baseline (T0) to long-term follow-up (T2). Number of patients for each group and timepoint are indicated.
Patient characteristics at 6–12 months’ follow-up
Patients still symptomatic while on a guideline-directed medical therapy underwent follow-up endomyocardial biopsy (figure 2). Virus-negative patients carrying the FOXO3A SNP rs12212067 showed a significant improvement in LVEF: ΔLVEF 13 % (95% CI 7.6 to 18.5) vs 6.5% (95% CI 4.3 to 8.7), p=0.013, SNP versus no SNP group, respectively (table 2), which resulted in significantly increased LVEF in carriers 50.9% (95% CI 45.4 to 56.3) vs 41.7% (95% CI 39.2 to 44.2), p=0.002 (figure 2A). The fractional shortening (FS) and LVEDD were not different, while LA diameters were significantly smaller in SNP carriers (p=0.028). These data implicate FOXO3A in the remodelling response in virus-negative patients with DCMi. FOXO3A has been shown to dampen immune cell function.7 11 To get more insight into the immune-modulating effects of FOXO3A, the extent of cardiac inflammation was determined in follow-up biopsies. As shown in table 2, there was a trend towards reduced inflammation in the overall SNP carrier cohort. Although no significant absolute numbers in specific inflammatory markers were determined, FOXO3A SNP rs12212067 carriers showed distinct changes in cardiac immune marker expression for: ΔMac-1+ (mm2) p=0.04; ΔCD45RO+ (mm2) p=0.034; ΔLFA-1+ (mm2) p=0.004 and ΔCD54+ (mm2) p=0.009, favouring the SNP carrier cohort. These data implicate FOXO3A activity in resolution of intracardiac inflammation in virus-negative patients. In line with these observations, SNP carriers showed significant improvement in clinical symptoms (figure 3).
Follow-up (6–12 months) characteristics according to genotype of FOXO3A SNP rs12212067 in patients with non-viral cardiomyopathy
Comparison of New York Heart Association class distribution by FOXO3A genotype over time. Comparison of the distribution of NYHA functional classification (shown as percentage occurrence) according to FOXO3A single-nucleotide polymorphism (SNP) status over time. The differences between No SNP and SNP carrier are shown at baseline, follow-up and long-term follow-up.
Patient characteristics at long-term follow-up (>24–36 months)
n=88 patients were followed up for more than 24 months. Patients’ characteristics are given in table 3. In line with our data characterising the disease state at 6–12 months, patients carrying the SNP were characterised by a significant improvement of LVEF: ΔLVEF 17.3% (95% CI 11.3 to 23.2) vs 9.4% (95% CI 5.6 to 13.2), p=0.033, SNP carriers versus non-carriers, respectively. LVEF was significantly enhanced in SNP carriers: 55.1% (95% CI 48.4 to 61.8) vs 46.7% (95% CI 42.9 to 50.4), p=0.030 (figure 2A). In accordance, reductions in LVEDD were significantly pronounced in this cohort (p=0.034). In line with these observations, LA size trended to be smaller and FS trended to be higher (table 3). In line with improvement of clinical symptoms, the rate of hospitalisations was significantly lower in carriers of the SNP (p=0.035, table 3).
Long-term follow-up (>24–36 months) characteristics according to genotype of FOXO3A SNP rs12212067 in patient with non-viral cardiomyopathy
With an exploratory intent, analyses with paired methods were used to account for changes in time per patient (online supplemental table 2).
Analysis of subgroups
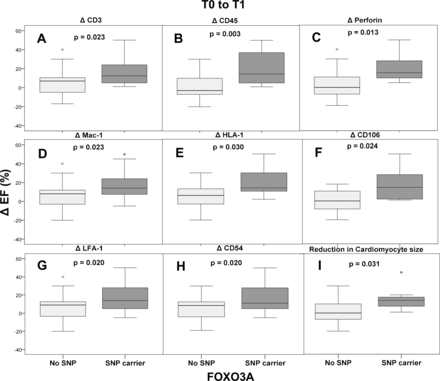
The extent of inflammation and its effect on improvement of left ventricular function depending on carrier status of SNP rs12212067 is depicted in figure 4. For the subgroup of patients characterised by decreasing numbers of cardiac perforin-positive cells during the disease course, a difference in LVEF of 3.9% (95% CI −1.4 to 9.2) without SNP vs 20.7% (95% CI 3.3 to 38.0) in SNP carriers (p=0.013) was depicted. In this line of evidence, the patient cohort with decreasing mononuclear infiltration, that is, with declining numbers of CD3+ cells and CD45RO+ cells at follow-up biopsies, showed a significant improvement of cardiac function (CD3+ cells/mm² with: ΔLVEF 5.3% (95% CI 1.0 to 9.7) without SNP vs 17.2% (95% CI 6.9 to 27.4) in SNP carriers (p=0.023) and CD45RO+ cells/mm² with: ΔLVEF 1.1% (95% CI −5.1 to 7.3) without SNP vs 20.2% (95% CI 7.0 to 33.4) in SNP carriers (p=0.003) as well as in patients with decreasing lymphocytic LFA-1+ expression (CD11a+) with: ΔLVEF 6.3% (95% CI 1.5 to 11.1) without SNP vs 17.9% (95% CI 7.5 to 28.4) in SNP carrier (p=0.02). Moreover, attenuation of monocyte marker Mac-1+ expression in the follow-up biopsies was associated with better improvement of LVEF in SNP carriers: ΔLVEF 6.4% (95% CI 2.0 to 10.8) without SNP vs 17.7% (95% CI 6.2 to 29.3) in SNP carriers (p=0.023), an effect, that was not significantly associated with age (online supplemental table 1).
Effects of intracardiac inflammation on improvement of left ventricular ejection fraction (LVEF) in relation to carrier status of FOXO3A single-nucleotide polymorphism (SNP) rs12212067. Patients with declining inflammation levels during follow-up were studied. Endomyocardial biopsy parameters were determined as follows: CD3+, CD45RO+, perforin+, Mac-1+, HLA-1+, CD106+, LFA-1+, CD54+ and cardiomyocyte diameter. Results are shown as boxplots with LVEF changes over time. EF, effect size.
In another subgroup with detectable decreased endothelial activation in follow-up biopsies, a significant improvement in LVEF was observed in SNP carriers, both for ICAM (intercellular adhesion molecule) CD54+ with: ΔLVEF 6% (95% CI 1.5 to 10.5) without SNP vs 17.2% (95% CI 6.6 to 27.9) in SNP carriers (p=0.02) as well as for VCAM (vascular cell adhesion molecule) CD106+ with: ΔLVEF 0.27% (95% CI −6.6 o 7.2) without SNP vs 18.3% (95% CI −0.98 to 37.7) in SNP carriers (p=0.024). Fittingly, the decreasing expression of HLA-1+ in cardiac tissue was also associated with the same significant effect: Δ LVEF 5.5% (95% CI 0.8 to 10.1) without SNP vs 21% (95% CI 3.8 to 38.2) in SNP carriers (p=0.03).
Similarly, reduction in cardiomyocyte size over time was associated with significant improvement in LVEF by SNP carriers: ΔLVEF 2% (95% CI −4.8 to 8.8) without SNP vs 15.7% (95% CI 2.4 to 29.0) in SNP-carriers (p=0.031).
Discussion
FOXO3A has been shown to act as a gatekeeper for homeostasis in different biological processes. In our study, virus-negative patients with cardiac inflammation and reduced ejection fraction carrying the gain of function SNP rs12212067 showed a better outcome characterised by enhanced improvement of LVEF, FS and attenuation of LVEDD/LVEDVI, and LA sizes. In follow-up biopsy specimens, this improvement over time was associated with significant reduction in inflammatory infiltrates within the myocardium and improvement of clinical symptoms.
Myocardial inflammation has been shown to be a predictor for outcome in inflammatory cardiomyopathy.12 Infections by cardiotropic viruses might trigger chronic immune processes frequently followed by heart-specific autoimmunity.13 Moreover, cardiac involvement in autoimmune disease has been observed.14 A breakdown in the control mechanisms protecting against autoimmune reactions by both, presentation of normally not accessible self-antigens and bystander-activation, induced by the pathogen, leads to the formation of autoreactive antibodies and T cells.15 Chronic autoimmune inflammation leads to cardiomyocyte destruction, reparative fibrosis and heart failure.16
Our previous data in pathogen-induced, that is, viral myocarditis, implicate the role of FOXO3A in differentiation and activation of NK cells.7 In an animal model of CVB3-induced myocarditis, lack of Foxo3a in mice resulted in enhanced NK cell activation, improved viral clearance, attenuated cardiac inflammation and preserved left ventricular function. In accordance with these findings, patients with a gain of function FOXO3A SNP rs12212067 were characterised by attenuation of NK cell function resulting in decreased viral clearance associated with prolonged cardiac inflammation and attenuated improvement of cardiac functional parameters suggesting a more severe course of virus-induced DCMi.7 These outcome data might be explained by viral persistence, ongoing inflammation and tissue injury in SNP carriers (ie, FOXO3A activation) due to diminished innate immune responses. In contrast, in the present study, non-carriers of the SNP were characterised by perforin persistence, a marker for NK cell accumulation, that was associated with decreased LV function and larger left ventricular end-diastolic diameters at follow-up indicating worse outcome in patients with virus-negative chronic (ie, autoimmune) cardiac inflammation. Carriers of the gain of function FOXO3A SNP, however, showed lower perforin levels and better outcome at follow-up (figure 5). These data are in accordance with observations of perforin as a marker cytokine for worse prognosis in DCMi.17 FOXO3A has been recently shown to act as an immunosuppressor.18 The attenuation of cardiac inflammation by FOXO3A might be caused by several immunological actions of the transcription factor. Besides modulating NK cell activity, FOXO3A has been shown to inhibit monocyte activation4 and phagocytic activity.4 Monocytes are modulators of innate and adaptive immune responses. In our study, the number of Mac1-positive cells delineating monocytes and macrophages was significantly reduced at 6 months follow-up in carriers of the SNP and associated with a significant increase in cardiac function at follow-up. Taken together, in contrast to pathogen-induced inflammation, where a robust inflammatory and innate immune response is warranted in order to clear the pathogen,8 virus-negative (ie, autoimmune) chronic inflammation might lead to unwarranted cell and tissue injury resulting in reduced LV function and worsening of heart failure symptoms.
Proposed effects of FOXO3A in cardiac inflammation. Summary of a working model of FOXO3A effects in cardiac inflammation: (A) in virus-induced inflammation, FOXO3A inhibits innate immune response by attenuating natural killer (NK) cell activation leading to delayed viral clearance and tissue injury.7 Virus-positive SNP carriers show reduced left ventricular ejection fraction (LVEF)/enlarged left ventricular end-diastolic diameter (LVEDD) and enhanced cardiac inflammatory activation at follow-up. (B) SNP carriers with virus-negative (autoimmune) cardiac inflammation show improved outcome due to immunosuppressive functions of FOXO3A. NK cell function and monocyte activation are reduced in SNP carriers.4 Inhibition of chronic cardiac inflammation leads to attenuated myocardial injury with positive remodelling resulting in better LVEF and reduced LVEDD.
Here, we provide further data for an immunosuppressive function of active FOXO3A in virus-negative DCMi. The SNP, a non-coding polymorphism in FOXO3A (rs12212067: T>G), does not lead to differential gene expression under unstimulated conditions but allele-specific expression occurs under inflammatory conditions leading to enhanced activity of the transcription factor. LFA-1 and CD3 are expressed on lymphocytes that characterise the adaptive immune response. Expression of both markers was attenuated at short-term and long-term follow-up in SNP carriers indicating resolution of inflammatory infiltrates associated with improvement of LV function and clinical symptoms as well as fewer hospitalisations. In accordance with the hypothesis of inhibition of chronic inflammation and attenuation of tissue injury, LV and atrial dimensions were significantly smaller in SNP carriers in line with observations of a milder course of chronic autoimmune diseases such as rheumatoid arthritis or chronic bowel disease.4 These observations are in contrast to viral cardiomyopathy7 or pathogen-induced inflammation such as malaria,4 where carriers of the SNP exhibited more severe disease and decreased clearance of pathogens.4 The dichotomy of FOXO3A action in pathogen-induced7 18 and autoimmune inflammation (our results18) is easy to ascertain. In virus infection, a strong innate immune response will be able to inhibit viral replication thereby preventing chronic inflammation and virus-inflicted tissue injury.9 Priming of the adaptive immune response will then clear the virus. In contrast, in autoimmune disease, tight control of innate and adaptive immune responses will diminish inflammatory cell-mediated tissue injury. There are still other mechanisms how FOXO3A activity is capable of attenuating the inflammatory process. FOXO3A modulates the function of T cells, regulatory T cell development and dendritic cell activation,19 and suppression of their activity by FOXO3A would attenuate the inflammatory process. FOXO3A can regulate the inflammatory and immune responses through targeting central transcription factors involved in self-tolerance such as NF-κB, as well as upregulating anti-inflammatory cytokines (eg, IL-10) while downregulating proinflammatory cytokines such as TNF-α in macrophages.4 These cytokines, together with antibodies against viral and cardiac proteins, further exacerbate the damage to the heart and impairment of systolic function due to changes of the contractile apparatus and matrix proteins.20–22 Taken together, several lines of evidence received from knockout studies in animals and associative studies in humans implicate FOXO3A in immunoregulation, homeostasis and attenuation of inflammation.
Endomyocardial biopsy is considered the gold standard for the diagnosis of acute or chronic inflammatory heart disease for identifying the aetiology of cardiac inflammation.23 MRI with T1 and T2 sequencing and late gadolinium enhancement is important for visualising structural changes, infiltration, inflammation, fibrosis and scarring. Although MRI provides non-invasive tissue characterisation, it is unable to identify infectious agents, or the degree and quality of inflammation but might be used for therapy monitoring if necessary.24 Immunosuppressive therapies have been shown to prevent later immune-mediated myocardial injury in patients with myocardial inflammation or persisting systemic autoimmunity despite virus elimination.25 26 Treatment approaches for these patients with post infectious chronic myocarditis/inflammatory cardiomyopathy consist of corticosteroids, azathioprine, mycophenolate, ciclosporin A or immunoadsorption with subsequent intravenous immunoglobulin therapy in addition to optimal heart failure medication.27 28
Modulation of FOXO3A activity might be a promising novel therapeutic approach, since activation of the transcription factor would benefit patients with autoimmune inflammation while inhibition of FOXO3A could provide viral clearance. The identification of the FOXO3A SNP might serve as one example for the combination of a genetic factor modulating the susceptibility, penetrance and severity of DCMi.
Several limitations apply to our study. First, although 221 patients were enrolled, only a small group of SNP rs12212067 carriers was investigated. Moreover, a multivariate analysis could only be performed in a subgroup of patients due to small patient numbers. However, it would have been difficult to recruit more patients with serial endomyocardial biopsy specimens for this disease entity given the rare distribution of the SNP in the whole cohort.
In conclusion, our study allows for new insights into genetically determined clinical course and eventual outcome or prognosis of cardiac inflammation. Enhancement of FOXO3A activity and resulting immunomodulation might afford protection in chronic autoimmune disease where inflammation should be suppressed but is less beneficial during infectious disease, where a proper immune response is needed to control rapid pathogen clearance and tissue injury. Further studies are needed to clarify the therapeutic potential of targeting FOXO3A activity with specific inhibitors presently in development.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the Institutional Ethics Committee (EK-No: 225-07, Charité University Hospital Berlin, Germany). Informed consent was obtained from all subjects.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
TT and CS are joint senior authors.
Twitter @TharusanT, @carsten.skurk
TT and CS contributed equally.
Contributors Conception and design: KM-Z, TT and C Skurk. Acquisition of data and analysis: KM-Z, FE, ZK, KK, H-PS, BH, WP, UL. Interpretation of data: KM-Z, TT, FE, GA, HP, UL, WP, C Scheibenbogen, C Skurk. Drafting the paper: KM-Z, ZK, HP, KK, GA. Revising the paper for important intellectual content: BH, WP, UL, C Scheibenbogen, TT, H-PS, FE, C Skurk. Final approval of the published version: KM-Z, FE, ZK, GA, HP, KK, H-PS, BH, WP, UL, C Scheibenbogen, TT, C Skurk. Agreement to be accountable for all aspects of the work: KM-Z, FE, ZK, GA, HP, KK, H-PS, BH, WP, UL, C Scheibenbogen, TT, C Skurk. Guarantor: C Skurk.
Funding This work was supported by German Research Foundation (DFG SK276/3-1 to C Skurk and C Scheibenbogen).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.