Abstract
Tirzepatide is a once-weekly GIP/GLP-1 receptor agonist. In this phase 3, randomized, open-label trial, insulin-naive adults (≥18 years of age) with type 2 diabetes (T2D) uncontrolled on metformin (with or without a sulphonylurea) were randomized 1:1:1:1 to weekly tirzepatide 5 mg, 10 mg or 15 mg or daily insulin glargine at 66 hospitals in China, South Korea, Australia and India. The primary endpoint was non-inferiority of mean change in hemoglobin A1c (HbA1c) from baseline to week 40 after treatment with 10 mg and 15 mg of tirzepatide. Key secondary endpoints included non-inferiority and superiority of all tirzepatide doses in HbA1c reduction, proportions of patients achieving HbA1c < 7.0% and weight loss at week 40. A total of 917 patients (763 (83.2%) in China) were randomized to tirzepatide 5 mg (n = 230), 10 mg (n = 228) or 15 mg (n = 229) or insulin glargine (n = 230). All doses of tirzepatide were non-inferior and superior to insulin glargine for least squares mean (s.e.) reduction in HbA1c from baseline to week 40: tirzepatide 5 mg, 10 mg and 15 mg, −2.24% (0.07), −2.44% (0.07) and −2.49% (0.07), respectively, and insulin glargine, −0.95% (0.07), with a treatment difference ranging from −1.29% to −1.54% (all P < 0.001). Proportions of patients achieving HbA1c < 7.0% at week 40 were greater in tirzepatide 5-mg (75.4%), 10-mg (86.0%) and 15-mg (84.4%) groups compared to insulin glargine (23.7%) (all P < 0.001). All tirzepatide doses led to superior body weight reduction at week 40: tirzepatide 5 mg, 10 mg and 15 mg, −5.0 kg (−6.5%), −7.0 kg (−9.3%) and −7.2 kg (−9.4%), respectively, compared to insulin glargine, 1.5 kg (+2.1%) (all P < 0.001). The most common adverse events with tirzepatide were mild to moderate decreased appetite, diarrhea and nausea. No severe hypoglycemia was reported. Tirzepatide demonstrated superior reductions in HbA1c versus insulin glargine in an Asia-Pacific, predominately Chinese, population with T2D and was generally well tolerated. ClinicalTrials.gov registration: NCT04093752.
Similar content being viewed by others
Main
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are both incretin hormones involved in glycemic control1. GLP-1 receptor agonists are an effective treatment option for patients with type 2 diabetes (T2D) that mimic the action of GLP-1 to stimulate insulin secretion and inhibit glucagon secretion in a glucose-dependent manner and also delay gastric emptying and decrease appetite2. GIP enhances post-prandial glucose-dependent insulin secretion by binding to its cognate receptor on pancreatic beta cells and mediates most of the incretin effect in healthy individuals3,4. GIP also stimulates glucagon secretion under euglycemic or hypoglycemic conditions and may also inhibit ectopic lipid accumulation and improve energy metabolism5,6.
Tirzepatide is a GIP/GLP-1 receptor agonist that consists of a 39-amino-acid polypeptide. It includes a C20 fatty di-acid moiety5,7, which confers a long half-life of ~5 d, enabling once-weekly administration8. In 2022, tirzepatide was approved in the United States, Europe and Japan for the treatment of T2D, supported by the findings of the global and Japanese SURPASS trials9,10,11,12,13,14,15. The results of the SURPASS trials have shown that tirzepatide provides clinically meaningful improvements in glycemic control and weight loss, with a favorable safety profile, in patients with different duration of disease and variety of background therapies compared to GLP-1 receptor agonists (semaglutide and dulaglutide), insulin degludec and insulin glargine9,10,11,12,13,14,15. Based on these findings, the latest consensus statement issued by the American Diabetes Association and the European Association for the Study of Diabetes categorizes tirzepatide as a therapy with the highest efficacy for both glycemic control and weight loss16.
Over 60% of individuals living with diabetes worldwide reside in Asia, and the prevalence of diabetes is increasing in this region17. The SURPASS 1–5 trials enrolled predominantly White patients, with Asians accounting for 3–30% of total participants in any given study. Subgroup analyses of Asian patients included in the SURPASS 1–5 trials and the two Japanese SURPASS trials (SURPASS J-mono and J-combo) show that tirzepatide has consistent outcomes in Asian patients compared with the findings in the overall study populations, who were predominantly White patients9,10,11,12,13,14,15. However, there is a need for further data on the efficacy and safety of tirzepatide in a broader range of Asian patient populations. In particular, there is currently a lack of data in Chinese patients, which is an important knowledge gap given that China is home to the highest number of individuals with diabetes of any country, accounting for more than one quarter of all individuals with diabetes globally17.
Metformin, with or without a sulphonylurea, is a widely used oral antihyperglycemic medication16, and basal insulin is the most commonly used injectable therapy in China for patients with T2D who are unable to achieve glycemic control through combinations of oral antihyperglycemic medications. However, although the addition of basal insulin to oral antihyperglycemic medications is highly effective for lowering blood glucose levels, it is also associated with hypoglycemia and weight gain, which can hamper adequate treatment intensification18.
This randomized, phase 3 trial was conducted to compare the effects of once-weekly tirzepatide 5 mg, 10 mg and 15 mg versus titrated once-daily insulin glargine on glycemic control and body weight in patients from the Asia-Pacific region (predominantly China) on stable doses of metformin, with or without a sulphonylurea and with inadequately controlled T2D.
Results
Patients
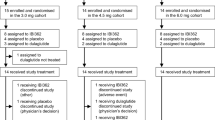
Patients were enrolled between 31 December 2019 and 27 January 2021, and the date of last follow-up was 24 November 2021. Of 1,232 patients screened, 917 were randomized (tirzepatide 5 mg: 230, tirzepatide 10 mg: 228, tirzepatide 15 mg: 229, insulin glargine: 230), and 907 received at least one dose of study medication and were included in the modified intention-to-treat (mITT) population (Fig. 1). Overall, 801 patients (87.4%) completed treatment; 815 patients (88.9%) completed the study; and 792 patients (86.4%) completed treatment without initiation of rescue antihyperglycemic therapy. The most common reasons for discontinuation of study treatment were adverse events (AEs) in the tirzepatide groups and patient withdrawal in the insulin glargine group.
Baseline characteristics were well balanced across the treatment groups (Table 1). Most patients were Asian (n = 892; 98.3%) and were enrolled in China (n = 756; 83.4%), South Korea (n = 104; 11.5%), India (n = 32; 3.5%) and Australia (n = 15; 1.7%). A total of 507 patients (55.9%) were male; the mean (s.d.) age was 54.1 (11.4) years; and the mean duration of diabetes was 7.65 (5.73) years. The mean (s.d.) baseline hemoglobin A1c (HbA1c) was 8.71% (0.96); body weight was 76.55 (14.48) kg; and body mass index (BMI) was 27.88 (4.02) kg m−2. Background oral antihyperglycemic medication therapy was metformin alone in 476 patients (52.5%) and metformin plus a sulphonylurea in 431 patients (47.5%).
Efficacy
Primary outcome
Tirzepatide led to greater reductions in HbA1c, from a mean baseline of 8.71%, compared to insulin glargine, with treatment differences observed from as early as week 4 and sustained until week 40 (Fig. 2a and Supplementary Table 1). The least squares (LS) mean (s.e.) reduction in HbA1c from baseline to week 40 was −2.44% (0.07) in the tirzepatide 10-mg group, −2.49% (0.07) in the tirzepatide 15-mg group and −0.95% (0.07) in the insulin glargine group (Table 2 and Fig. 2b). The primary endpoint (non-inferiority of tirzepatide 10 mg and/or 15 mg to insulin glargine at week 40) was met, that estimated treatment difference compared to insulin glargine was −1.49% (multiplicity-adjusted 97.5% confidence interval (CI) −1.72 to −1.26) for tirzepatide 10 mg and −1.54% (−1.77 to −1.31) for tirzepatide 15 mg.
a, LS mean HbA1c values over time. b, LS mean changes in the HbA1c level from baseline at week 40 were estimated using an MMRM without missing value imputation in patients who received at least one dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220). Statistical tests for 10 mg and 15 mg were two-sided at a significance level of 0.025; statistical tests for 5 mg were two-sided at a significance level of 0.05; and adjustments were made for multiplicity. c, Patients achieving HbA1c < 7.0%, ≤6.5% and <5.7% target attainment were estimated using logistic regression analysis with missing value imputed by an MMRM using efficacy analysis set at 40 weeks (5 mg, n = 228; 10 mg, n = 222; 15 mg, n = 224; insulin glargine, n = 215). Statistical tests for patients achieving HbA1c < 7.0% were two-sided at a significance level of 0.05, and adjustments were made for multiplicity. All other statistical tests were two-sided at a significance level of 0.05, and no adjustments were made for multiplicity. d, LS mean FSG values over time. e, LS mean changes from baseline in FSG at week 40 were estimated using an MMRM without missing value imputation in patients who received at least one dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220). Statistical tests were two-sided at a significance level of 0.05, and no adjustments were made for multiplicity. f, LS mean seven-point SMBG at baseline and week 40. Dashed lines indicate treatment targets. Error bars indicate s.e. n, number of patients achieving target with missing value imputed by an MMRM; N, number of patients who were randomized and received at least one dose of study drug; No., number of patients with baseline and post-baseline value at the specified timepoint.
Key secondary outcomes
The LS mean (s.e.) reduction in HbA1c from baseline to week 40 was −2.24% (0.07) in the tirzepatide 5-mg group. The estimated treatment difference for tirzepatide 5 mg was −1.29% (95% CI −1.49 to −1.09), which also met the criteria for non-inferiority. In addition, all three tirzepatide doses demonstrated superiority in LS mean reduction in HbA1c from baseline to week 40 compared to insulin glargine (P < 0.001 for all comparisons) (Table 2 and Fig. 2b). The daily mean insulin dose at week 40 was 25.3 U (s.d. 11.1; 0.33 U kg−1 (s.d. 0.14)). ANCOVA analysis in the full analysis set (FAS) also showed a significantly greater reduction in HbA1c at week 40 with all three doses of tirzepatide versus insulin glargine (Extended Data Fig. 1a).
The proportion of patients achieving HbA1c < 7.0% at week 40 was significantly greater in the tirzepatide 5-mg (75.4%), 10-mg (86.0%) and 15-mg (84.4%) groups compared to the insulin glargine group (23.7%) (P < 0.001 for all comparisons) (Fig. 2c). ANCOVA analysis in the FAS also showed a significantly higher proportion of patients achieving HbA1c < 7.0% at week 40 with all three doses of tirzepatide versus insulin glargine (Extended Data Fig. 1b).
Significant reductions in body weight were observed in the tirzepatide groups versus the insulin glargine group from week 4 onwards, with numerically greater reductions in the 10-mg and 15-mg groups after dose escalation from week 8 and not appearing to plateau by week 40 (Fig. 3a and Supplementary Table 2). By week 40, the LS mean body weight decreased by −5.0 kg (−6.5%) with tirzepatide 5 mg, −7.0 kg (−9.3%) with tirzepatide 10 mg and −7.2 kg (−9.4%) with tirzepatide 15 mg, whereas it increased by 1.5 kg (+2.1%) in the insulin glargine group (P < 0.001 versus baseline in each group), giving an estimated treatment difference of −8.5% to −11.5% (P < 0.001 for all comparisons; Table 2 and Fig. 3b,c). ANCOVA analysis in the FAS also showed a significantly greater reduction in body weight at week 40 with all three doses of tirzepatide versus insulin glargine (Extended Data Fig. 2).
a, LS mean body weight over time. b, LS mean changes from baseline in body weight at week 40 were estimated using an MMRM without missing value imputation in patients who received at least one dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220). Statistical tests for 10 mg and 15 mg were two-sided at a significance level of 0.025; statistical tests for 5 mg were two-sided at a significance level of 0.05; and adjustments were made for multiplicity. c, LS mean percent changes from baseline in body weight over time. d, Body weight loss target attainment was estimated using logistic regression analysis with missing value imputed by an MMRM using efficacy analysis set at 40 weeks (5 mg, n = 228; 10 mg, n = 222; 15 mg, n = 224; insulin glargine, n = 215). Statistical tests were two-sided at a significance level of 0.05, and no adjustments were made for multiplicity. e, LS mean BMI over time. f, Estimate mean changes from baseline in fasting lipid profile at week 40 were estimated using an MMRM without missing value imputation in patients who received at least one dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220). Statistical tests were two-sided at a significance level of 0.05, and no adjustments were made for multiplicity. Error bars indicate s.e. n, number of patients achieving target with missing value imputed by an MMRM; N, number of patients who were randomized and received at least one dose of study drug; No., number of patients with baseline and post-baseline value at the specified timepoint.
Additional secondary outcomes
The proportion of patients achieving HbA1c ≤ 6.5% and <5.7% was also significantly greater in each tirzepatide group versus insulin glargine (Table 2).
Fasting serum glucose (FSG) decreased from baseline to week 40 in all four treatment groups (P < 0.001 versus baseline) (Fig. 2d). LS mean reductions in FSG were significantly greater for tirzepatide 5 mg (−3.25 mmol L−1), 10 mg (−3.68 mmol L−1) and 15 mg (−3.60 mmol L−1) compared to insulin glargine (−2.57 mmol L−1) (P < 0.001 for all tirzepatide doses versus insulin glargine) (Fig. 2e).
All three tirzepatide doses led to significantly greater reductions versus insulin glargine in overall daily mean, pre-meal daily mean and 2-h post-meal daily mean self-measured blood glucose (SMBG) values at week 40 (Table 2). Week 40 SMBG values were significantly lower with all three tirzepatide doses than with glargine at all timepoints, except for tirzepatide 5 mg at morning pre-meal, which showed a similar reduction to insulin glargine (Fig. 2f). Furthermore, the LS mean (s.e.) 2-h post-prandial glucose levels were close to 7.8 mmol L−1 (the normal range for post-meal glucose) at week 40 in patients receiving tirzepatide 10 mg (7.88 mmol L−1 (0.146)) and 15 mg (7.77 mmol L−1 (0.147)). All doses of tirzepatide significantly reduced mean pre-meal to 2-h post-meal SMBG excursions compared to insulin glargine at week 40 (Table 2).
A significantly higher proportion of patients in each tirzepatide group achieved ≥5%, ≥10% and ≥15% weight loss compared to insulin glargine (Fig. 3d).
Compared to patients receiving insulin glargine, those in the tirzepatide groups had significantly higher total Diabetes Treatment Satisfaction Questionnaire status (DTSQc) scores at week 40, indicating greater improvements in satisfaction than those receiving insulin glargine (Supplementary Table 3).
Safety
Serious AEs were reported by 6.1–6.6% of patients in the tirzepatide groups and 9.1% of patients in the insulin glargine group (Supplementary Table 4). There were three deaths during the study, all considered unrelated to study medication by the investigators: two in the insulin glargine group (sudden cardiac death and death due to car accident) and one in the tirzepatide 15-mg group (sudden death 28 d after completing study treatment). The most common treatment-emergent adverse events (TEAEs) among patients receiving tirzepatide were gastrointestinal related (diarrhea, nausea and vomiting) and decreased appetite, which were most frequent during the dose-escalation period and decreased afterwards (Extended Data Fig. 3). Most of these TEAEs were mild to moderate in severity. Among the tirzepatide treatment groups, the median duration of diarrhea was 3 d, and the median durations of nausea and vomiting ranged from 3 d to 4 d and from 1 d to 2 d, respectively (Supplementary Table 5). Treatment discontinuations due to AEs occurred in a higher proportion of patients in the tirzepatide 10-mg (13.2%) and 15-mg (12.2%) groups than in the tirzepatide 5-mg (4.3%) and insulin glargine groups (2.7%) (Table 3). Among all patients receiving tirzepatide (n = 687), the most common AEs resulting in treatment discontinuation were decreased appetite (2.3%), nausea (2.3%) and diarrhea (1.5%) (Table 3).
No severe hypoglycemia was reported during the study in either treatment group. The incidence of documented hypoglycemia at <3.0 mmol L−1 (<54 mg dl−1) was low in the tirzepatide (3.9–4.8%) and glargine (4.1%) groups. The incidence of documented hypoglycemia at ≤3.9 mmol L−1 (≤70 mg dl−1) was lower with tirzepatide (27.4–32.9%) versus glargine (44.1%). More patients receiving a sulfonylurea reported hypoglycemic events at both hypoglycemia levels (Table 3). Only two patients (0.3%) receiving tirzepatide (both in the 5-mg group) were prescribed rescue medication for persistent hyperglycemia compared to eight (3.6%) in the insulin glargine group.
No pancreatitis was reported in the tirzepatide groups, and one patient (0.5%) in the glargine group had adjudication-confirmed pancreatitis. The mean levels of p-amylase and lipase increased in patients treated with tirzepatide and decreased toward baseline levels during the safety follow-up (Extended Data Fig. 4a,b). The incidence of cholelithiasis was 0.4–0.9% across the tirzepatide groups and 0.5% in the glargine group. Acute cholecystitis was reported in two patients receiving tirzepatide (10 mg, n = 1 (0.4%) and 15 mg, n = 1 (0.4%)). No medullary thyroid carcinoma or C-cell hyperplasia events were reported. There were no clinically relevant changes in mean calcitonin values in the tirzepatide groups during the treatment period. Malignancies were reported in one (0.4%) patient in the tirzepatide 5-mg group, two (0.9%) in the 10-mg group, two (0.9%) in the 15-mg group and one (0.5%) in the insulin glargine group. There was no clear trend in the incidence of malignancies with respect to treatment assignment. Treatment-emergent potential diabetic retinopathy was reported in one patient each in the tirzepatide 5-mg (0.4%), tirzepatide 15-mg (0.4%) and glargine (0.5%) groups and three patients in the tirzepatide 10-mg (1.3%) group. A total of nine adjudication-confirmed major adverse cardiovascular events (MACE-4) were reported in eight patients (5 mg, n = 3; 10 mg, n = 1; 15 mg, n = 4) in the tirzepatide groups and one patient in the insulin glargine group. At week 40, patients in the tirzepatide groups showed increases in pulse rate (2.2–4.8 beats per minute (bpm)), whereas no significant changes in pulse rate were observed in the insulin glargine group (Supplementary Table 6). In addition, at week 40, patients in the tirzepatide groups showed significant decreases in systolic blood pressure (SBP; −6.7 to −7.3 mmHg) and diastolic blood pressure (DBP; −3.4 to −4.0 mmHg) compared to baseline (Supplementary Table 6). No changes in blood pressure were observed in the insulin glargine group.
The incidence of potential hypersensitivity reactions was similar and low across the tirzepatide (3.3% (23/687)) and insulin glargine (2.3% (5/220)) groups, and no severe or serious hypersensitivity reactions were reported (Table 3). Mild to moderate injection site reactions were reported by 2% (14/687) of patients receiving tirzepatide and 1.4% (3/220) of patients receiving insulin glargine.
Exploratory outcomes
A higher proportion of patients in the tirzepatide groups achieved the composite endpoints of HbA1c < 7.0% (or ≤6.5%) without weight gain (or HbA1c < 7.0% with weight loss ≥5%) and without documented symptomatic hypoglycemia (<3.0 mmol L−1 (<54 mg dl−1)) or severe hypoglycemia (Table 2).
Both BMI and waist circumference decreased significantly from baseline in all tirzepatide groups from week 4 onwards (Fig. 3e and Extended Data Fig. 5). At week 40, patients receiving tirzepatide 5 mg (26.1 kg m−2), 10 mg (25.3 kg m−2) and 15 mg (25.3 kg m−2) had a significantly reduced BMI compared to baseline (27.9 kg m−2), in contrast to an increased BMI in patients receiving insulin glargine (28.5 mg kg−2) (P < 0.001 for each tirzepatide group versus insulin glargine) (Fig. 3e). Similarly, the LS mean waist circumference decreased by −5.8 cm, −7.3 cm and −7.6 cm with tirzepatide 5 mg, 10 mg and 15 mg, respectively, whereas it increased by 0.8 cm in the insulin glargine group (P < 0.001 for each tirzepatide group versus insulin glargine) (Extended Data Fig. 5).
Tirzepatide was also associated with other metabolic improvements. Compared to insulin glargine, all three tirzepatide doses led to significantly greater reductions from baseline to week 40 in triglycerides (−25.8% to −29.8%), total cholesterol (−4.16% to −4.77%), non-high-density lipoprotein cholesterol (non-HDL-C) (−8.08% to −9.63%) and very-low-density lipoprotein cholesterol (VLDL-C) (−24.3% to −28.6%) and significantly greater increases in HDL-C (5.13–8.51%) (Fig. 3f), whereas baseline and post-baseline usage of lipid-lowering medications was balanced across the treatment groups (Supplementary Table 7). Larger reductions in mean alanine transaminase (ALT) and aspartate transaminase (AST) levels from baseline to week 40 were observed among patients in the tirzepatide 5-mg (−31.0% and −17.3%), 10-mg (−34.1% and −19.8%) and 15-mg (−35.2% and −20.8%) dose groups compared to those in the insulin glargine group (−14.8% and −5.7%) (Extended Data Fig. 6a,b). Greater reductions in urine albumin/creatinine ratio (UACR) from baseline to week 40 were observed in the tirzepatide groups (−37.3% to −48.5%) compared to the insulin glargine group (−9.8%) (P < 0.001 for all tirzepatide groups versus insulin glargine) (Supplementary Table 8).
Pre-specified subgroup analyses
Subgroup analysis revealed consistent HbA1c reductions across the three tirzepatide dose levels among patients receiving metformin alone or metformin plus a sulphonylurea and between patients enrolled in China versus other countries (P value for treatment-by-subgroup interaction: P = 0.712 and P = 0.124, respectively) (Extended Data Fig. 7).
All tirzepatide doses had a consistent effect on body weight across the subgroups of patients receiving metformin alone or metformin plus a sulphonylurea and those enrolled in China versus other countries (P value for treatment-by-subgroup interaction: P = 0.547 and P = 0.497, respectively) (Extended Data Fig. 8).
Discussion
Consistent with the results reported in the SURPASS 1–5 and SURPASS-J trials, the SURPASS-AP-Combo trial confirms the efficacy and safety of tirzepatide in a predominantly Chinese Asia-Pacific population with T2D9,10,11,12,13,14,15. The primary endpoint of this study—non-inferiority of once-weekly tirzepatide 10 mg and/or 15 mg to insulin glargine at week 40—was met.
Our results also show that weekly tirzepatide 5 mg, 10 mg and 15 mg led to superior glycemic control compared to titrated daily insulin glargine, as demonstrated by superior reductions in HbA1c levels (−2.24% to −2.49%), which is consistent with the global SURPASS 1–5 and two Japanese SURPASS trials (−1.87% to −3.0%)9,10,11,12,13,14,15. Patients receiving tirzepatide also achieved higher rates of HbA1c < 7%, ≤6.5% and <5.7%, a greater reduction in FSG and a reduction of 2-h post-prandial glucose to near-normal values over 40 weeks of treatment. In China, previous estimates have revealed that only around 49.2% of patients with T2D who are receiving treatment achieve the glycemic control target of HbA1c < 7%19, compared to 75–86% of patients receiving tirzepatide in the present study. Furthermore, up to 80.8% of patients receiving tirzepatide achieved the composite endpoints HbA1c < 7% without weight gain and without clinically significant documented symptomatic hypoglycemia or severe hypoglycemia, which indicates that tirzepatide improves glycemic control in a safe manner. Pre-specified subgroup analyses showed consistent, superior reductions in HbA1c and weight loss with tirzepatide versus insulin glargine, regardless of baseline sulphonylurea use and across patients enrolled in China and outside of China.
As reported in a previous phase 1 study, tirzepatide improves first-phase and second-phase insulin secretion and decreases glucagon secretion, and both mechanisms occur in a glucose-dependent manner20. In addition, tirzepatide results in improvements in insulin sensitivity, which may contribute to the overall high antihyperglycemic efficacy with a low risk of hypoglycemia compared to basal insulin, as demonstrated here.
By week 40, patients in the insulin glargine group were receiving a mean insulin dose of 0.33 U kg−1 d−1, which is similar to reports from multiple phase 3 trials of insulin glargine using the same treat-to-target algorithm as the present study in insulin-naive Chinese patients with T2D (around 0.3 U kg−1 d−1)21,22,23,24. This finding suggests that the insulin glargine dose titration and overall dose levels used in the SURPASS-AP-Combo are representative of similar clinical trials in this patient population, although lower than reported in global trials (around 0.5 U kg−1 d−1)10,12,25,26. In addition, although the SURPASS-4 trial compared tirzepatide to insulin glargine, it was conducted in patients with increased cardiovascular risk10, whereas our study was conducted in a broader T2D population regardless of cardiovascular risk.
Overweight or obesity is associated with a higher risk of developing T2D and diabetes complications and more difficulty achieving glycemic control27. Therefore, weight reduction is a central component of T2D management. The prevalence of overweight and obesity among individuals in Asian countries is high, with an estimated prevalence of overweight among adults (females/males) ranging from 23.9%/19.0% in India28 to 28.1%/25.1% in South Korea29. The results of the Chinese National Diabetes and Metabolic Disorders survey estimated that 65.3% of Chinese individuals with T2D are either with obesity or overweight30,31. The 2020 Chinese Diabetes Society guidelines recommend reduction of body weight by at least 5%, and the 2021 Consensus of Chinese Experts on the Remission of Type 2 Diabetes Mellitus recommends reduction of body weight by at least 10% for remission of diabetes19,32. Here we show that tirzepatide leads to reductions in body weight of −5.0 kg (−6.5%) to −7.2 kg (−9.4%), and up to 74.1% and 45.1% of patients achieved weight reductions of ≥5% and ≥10%, respectively, over 40 weeks. Tirzepatide-induced weight loss is predominantly driven by fat mass loss and hypothesized to not only be mediated by appetite suppression, although further research is ongoing to investigate the mechanism of action33. In addition, the ongoing SURMOUNT-CN trial (NCT05024032) will further investigate the weight reduction effects of tirzepatide in a Chinese population with obesity.
In addition to weight loss, tirzepatide reduced waist circumference and BMI (versus an expected increase with insulin glargine) as well as provided other metabolic benefits, including greater reductions in triglycerides, total cholesterol, non-HDL-C and VLDL-C; an increase in HDL-C; reductions in SBP and DBP; and reductions in ALT and AST levels and UACR, which are known risk factors for cardiovascular disease and non-alcoholic fatty liver disease. These findings suggest that tirzepatide may potentially provide a benefit for the long-term outcomes of diabetes comorbidities34,35.
As expected, based on the results of the completed SURPASS trials, gastrointestinal AEs were the most common side effects of tirzepatide in the present study, which is consistent with the safety profile of GLP-1 receptor agonists9,10,11,12,13,36. Although a higher incidence of gastrointestinal AEs was reported in comparison to the global and Japanese tirzepatide trials9,10,11,12,13,14,15, the severity of events and discontinuation rates were consistent with previous reports, and treatment satisfaction increased with tirzepatide, which indicates that these AEs were not a barrier to successful treatment.
One of the primary strengths of the present study is that most patients were enrolled in China, which is home to the largest number of individuals with T2D in the world. The data are, therefore, of high relevance to a large patient population17. Secondly, the study had a large sample size, and a high proportion of participants completed treatment and completed the trial, giving very complete data. Therefore, the results of this study have high generalizability to clinical practice in China. The study had several limitations, including the open-label study design; however, it was not feasible to mask treatment assignment due to differences in the frequency of administration, injection devices, insulin titration scheme and tirzepatide dose-escalation schemes. In addition, the study had a relatively short duration of 40 weeks, and a longer study duration may have provided further insights on the full weight loss effects of tirzepatide. In this study population, there was no clear dose-dependent efficacy for HbA1c and body weight reduction between the tirzepatide 10-mg and 15-mg groups. Further studies of tirzepatide in Chinese and Asian patients may assist the interpretation of this observation.
In summary, the results of this phase 3 SURPASS-AP-Combo trial show that weekly tirzepatide significantly improved glycemic control in a predominantly Chinese Asia-Pacific population of patients with T2D inadequately controlled on metformin, with or without sulphonylurea. The study also showed that the safety profile of tirzepatide was consistent with the GLP-1 receptor agonist class.
Methods
Inclusion and ethics
Ethics review boards at each study site approved the protocol (see Supplementary Table 9 for the full list). The trial was conducted in accordance with the principles of international ethics guidelines, including the Declaration of Helsinki, and applicable laws and regulations. Written informed consent was obtained from each patient before any study procedures.
Study design
This 40-week, multicenter, randomized, phase 3, open-label, parallel-group trial was conducted at 66 medical research centers and hospitals in China (n = 43), South Korea (n = 13), Australia (n = 6) and India (n = 4). The study consisted of a 1-week screening period, a 2-week lead-in period, a 40-week treatment period and a 4-week safety follow-up period (Extended Data Fig. 9).
Randomization and masking
Patients were recruited and enrolled at the 66 study sites by investigators and randomly assigned 1:1:1:1 to tirzepatide 5 mg, 10 mg, 15 mg or insulin glargine using a computer-generated randomization sequence, which was implemented via an interactive web-response system (IWRS). Randomization was stratified according to baseline HbA1c (≤8.5% versus >8.5%), country and oral antihyperglycemic medication use (metformin alone versus metformin plus a sulphonylurea). An open-label design was chosen owing to the different dosing frequencies, titration schemes and injection devices of insulin glargine compared to tirzepatide, which precluded masking of treatment allocation.
Procedures
Tirzepatide was administered once a week by subcutaneous injection using single-dose pens. The starting tirzepatide dose for all patients was 2.5 mg, which was increased by 2.5 mg every 4 weeks until the target dose was reached. If intolerable gastrointestinal side effects occurred and persisted despite advice to eat smaller meals, symptomatic treatment and omission of one tirzepatide dose, the tirzepatide dose could be de-escalated to a lower, tolerated maintenance dose (5 mg or 10 mg), which was then continued for the remainder of the study. Dose de-escalation was not permitted after week 24.
Insulin glargine was administered once daily at bedtime by subcutaneous injection using a pre-filled pen. The starting dose was 6 IU d–1, which was then titrated using a treat-to-target algorithm to reach the fasting blood glucose target of 4.0–5.6 mmol L−1 based on SMBG levels (Supplementary Table 10).
All patients continued background therapy with metformin, with or without a sulphonylurea, at the same pre-study dose. Patients with severe, persistent hyperglycemia meeting pre-defined criteria (Supplementary Information, page 14) could initiate rescue antihyperglycemic therapy.
Patients
This study included insulin-naive adults (≥18 years of age) with T2D inadequately controlled (HbA1c ≥ 7.5% and ≤11.0%) despite stable treatment with metformin, with or without a sulphonylurea, for ≥2 months. Patients were required to have a BMI of ≥23 kg m−2 and stable body weight during the previous 3 months before enrollment.
Exclusion criteria were as follows: type 1 diabetes; estimated glomerular filtration rate ≤45 ml min−1/1.73 m2; history of pancreatitis, proliferative diabetic retinopathy, diabetic maculopathy and non-proliferative diabetic retinopathy requiring acute treatment; history of severe hypoglycemia and/or hypoglycemia unawareness within the last 6 months; history of ketoacidosis or hyperosmolar state/coma; known clinically significant gastric emptying abnormality; having undergone or planning to undergo a gastric bypass surgery; restrictive bariatric surgery; or chronic use of drugs that directly affect gastrointestinal motility. Patients were not permitted to have any of the following conditions within the last 2 months: acute myocardial infarction; cerebrovascular accident; hospitalization due to congestive heart failure; New York Heart Association Functional Classification III or IV congestive heart failure; acute or chronic hepatitis or any other liver disease other than non-alcoholic fatty liver disease; ALT level more than 3.0 times the upper limit of the reference range; evidence of a significant, uncontrolled endocrine abnormality or family/personal history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2; a serum calcitonin level of ≥35 ng L−1; known or suspected hypersensitivity to the study drug or related drugs; evidence of a significant active autoimmune abnormality that is likely to require concurrent treatment with systemic glucocorticoids in the next 12 months; a transplanted organ or awaiting an organ transplant; a history of active or untreated malignancy; remission from a clinically significant malignancy (other than basal or squamous cell skin cancer, in situ carcinomas of the cervix or in situ prostate cancer) for less than 5 years; a history of any other condition that may preclude the patient from following and completing the protocol; or any hematological condition that may interfere with HbA1c measurement. Patients who had received any of the following therapies in the past 3 months and/or between study entry and randomization were also excluded: any glucose-lowering agent other than metformin or sulphonylurea; prescription drugs that promote weight loss; and chronic (>2 weeks or 14 d) systemic glucocorticoid therapy (excluding topical, intraocular, intranasal or inhaled preparations). Further details of the inclusion and exclusion criteria are provided in Supplementary Information (pages 14–18).
Outcomes and assessments
The primary study objective was non-inferiority of tirzepatide 10 mg or 15 mg or both compared to insulin glargine for the mean change in HbA1c from baseline to week 40 at a non-inferiority margin (NIM) of 0.4%. Key secondary objectives, controlled for type I error, were to demonstrate non-inferiority of tirzepatide 5 mg versus insulin glargine for the mean change in HbA1c from baseline to week 40; superiority of each tirzepatide dose versus insulin glargine for weight loss from baseline to week 40; superiority of each tirzepatide dose versus insulin glargine for the mean change in HbA1c from baseline to week 40; and superiority of each tirzepatide dose versus insulin glargine for the proportion of patients achieving HbA1c < 7.0% at week 40. Additional secondary objectives (not controlled for type I error) were the mean change from baseline in FSG; the proportion of patients achieving HbA1c ≤ 6.5% and <5.7%; the mean change from baseline in daily average glucose from SMBG profiles; the proportion of patients attaining weight loss of ≥5%, ≥10% and ≥15% from baseline; and patient-reported satisfaction with treatment, assessed using the Diabetes Treatment Satisfaction Questionnaire status (DTSQs) and Diabetes Treatment Satisfaction Questionnaire change (DTSQc). Exploratory endpoints were change in BMI, waist circumference and lipids (total cholesterol, HDL-C, non-HDL-C, VLDL-C and triglycerides) from baseline to week 40.
Safety endpoints included the incidence of TEAEs, serious AEs and discontinuation of study treatment due to AEs. AEs of special interest included hypoglycemia, severe persistent hyperglycemia, thyroid safety, gastrointestinal disorders, diabetic retinopathy, MACE-4, allergic/hypersensitivity reactions and adjudicated pancreatic AEs. An independent committee adjudicated pancreatic AEs and MACE-4. Further safety endpoints included change from baseline in vital signs (SBP, DBP and pulse rate).
HbA1c was analyzed using Bio-Rad Variant II. FSG, lipids, pancreatic amylase, lipase and hepatic enzymes were analyzed using Roche Cobas C501. All of these tests were performed by central laboratories (Q Squared Solutions, Beijing, China, for samples collected from China, and Q Squared Solutions, Singapore, for samples collected from the other countries). Weight and waist circumference were measured at fasting visits.
Statistical analysis
This study was designed to randomly assign patients (1:1:1:1) to tirzepatide 5 mg, 10 mg or 15 mg or insulin glargine, respectively. The sample size calculation assumed at least a 0.45% superior HbA1c reduction with tirzepatide 10 mg and 15 mg versus insulin glargine, a common s.d. of 1.2% and no more than 25% initiation of rescue therapy or discontinuation from study drug. Given these assumptions, enrollment of approximately 956 patients would provide a 90% power to demonstrate the superiority of all tirzepatide doses compared to insulin glargine at a two-sided significance level of 0.05. This sample size calculation provided a more than 99% power for the non-inferiority comparison with a NIM of 0.4%. This NIM was in line with the requirements of the China National Medical Products Administration for clinical trials of novel treatments for T2D37. A graphical multiple-testing procedure was employed to control the type I error for the primary and key secondary endpoint analyses (Extended Data Fig. 10).
Change in HbA1c was analyzed using a mixed model for repeated measures (MMRM) with the dependent variable of change from baseline, independent variables of baseline value, treatment group, visit, treatment-by-visit interaction, country and sulphonylurea use as covariates and an unstructured covariance structure. MMRMs were also used to analyze changes in body weight, BMI, waist circumference, FSG and daily average SMBG values. Changes in lipid profile were evaluated by MMRM analysis using log-transformation. HbA1c target attainment was analyzed by logistic regression with treatment, country, sulphonylurea use and baseline HbA1c value as covariates. Logistic regression was also used to evaluate FSG and weight loss target attainment. For analyses of HbA1c and weight loss target attainment, missing values were imputed using the predicted values from MMRM analysis.
For evaluation of treatment adherence, patients still receiving study drug at the endpoint visit were regarded as treatment completers, regardless of temporary interruptions or the treatment actually received. Owing to the Coronavirus Disease 2019 (COVID-19) pandemic during the study, the endpoint visit window may have been adjusted to week 38–44, as defined in the protocol amendment.
The primary analysis and other efficacy measures were evaluated in the efficacy analysis set, defined as data obtained during the treatment period from all randomized patients who received ≥1 dose of study drug (mITT population), excluding data collected after initiating rescue antihyperglycemic medication or discontinuation from study drug. A supplementary analysis of efficacy endpoints was conducted using ANCOVA with multiple imputation of missing primary measures, using data from the FAS, defined as data obtained during treatment from the mITT population, regardless of adherence to study drug or initiation of rescue antihyperglycemic medication. Safety analyses used all data collected from the start of treatment to the end of the safety follow-up period in the mITT population.
The InForm Electronic Data Capture platform was used for study data collection. All statistical analyses were conducted using SAS version 9.4 or higher software. The study protocol was registered at ClinicalTrials.gov (NCT04093752).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data from the analyses in this study cannot be publicly available due to the sponsor’s (Eli Lilly and Company) contractual obligations. Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and the European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at https://vivli.org/.
Code availability
No customer code was used for data analysis in this study.
References
Nauck, M. A., Quast, D. R., Wefers, J. & Pfeiffer, A. F. H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes. Metab. 23, 5–29 (2021).
Nauck, M. A., Quast, D. R., Wefers, J. & Meier, J. J. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol. Metab. 46, 101102 (2021).
Gasbjerg, L. S. et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides 125, 170183 (2020).
Baggio, L. L. & Drucker, D. J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 46, 101090 (2021).
Min, T. & Bain, S. C. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 12, 143–157 (2021).
Samms, R. J., Coghlan, M. P. & Sloop, K. W. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol. Metab. 31, 410–421 (2020).
Frias, J. P. et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392, 2180–2193 (2018).
Papachristou, S., Popovic, D. S. & Papanas, N. The new dual gastric inhibitory peptide/glucagon-like peptide 1 agonist tirzepatide in type 2 diabetes: is the future bright? Diabetes Metab. Res. Rev. 37, e3503 (2021).
Dahl, D. et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 327, 534–545 (2022).
Del Prato, S. et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 398, 1811–1824 (2021).
Frías, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Ludvik, B. et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 398, 583–598 (2021).
Rosenstock, J. et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398, 143–155 (2021).
Inagaki, N., Takeuchi, M., Oura, T., Imaoka, T. & Seino, Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 10, 623–633 (2022).
Kadowaki, T., Chin, R., Ozeki, A., Imaoka, T. & Ogawa, Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 10, 634–644 (2022).
Davies, M. J. et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45, 2753–2786 (2022).
IDF Diabetes Atlas, 10th edition. International Diabetes Federation https://www.diabetesatlas.org (2021).
Niswender, K. D. Basal insulin: physiology, pharmacology, and clinical implications. Postgrad. Med. 123, 17–26 (2011).
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes Mellit. 13, 315–409 (2021).
Heise, T. et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 10, 418–429 (2022).
Feng, W., Chen, W., Jiang, S., Du, L. & Zhu, D. Efficacy and safety of LY2963016 insulin glargine versus insulin glargine (Lantus) in Chinese adults with type 2 diabetes: a phase III, randomized, open-label, controlled trial. Diabetes Obes. Metab. 23, 1786–1794 (2021).
Ji, L. et al. Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naïve people with type 2 diabetes: the EDITION AP randomized controlled trial. Diabetes Obes. Metab. 22, 612–621 (2020).
Ji, L. et al. Higher versus standard starting dose of insulin glargine 100 U/mL in overweight or obese Chinese patients with type 2 diabetes: results of a multicentre, open-label, randomized controlled trial (BEYOND VII). Diabetes Obes. Metab. 22, 838–846 (2020).
Wang, W. et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52-week open-label, randomized phase III trial. Diabetes Obes. Metab. 21, 234–243 (2019).
Aroda, V. R. et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 5, 355–366 (2017).
Pozzilli, P. et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes. Metab. 19, 1024–1031 (2017).
Aras, M., Tchang, B. G. & Pape, J. Obesity and diabetes. Nurs. Clin. North Am. 56, 527–541 (2021).
Hills, A. P. et al. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 6, 966–978 (2018).
Jang, M. & Berry, D. Overweight, obesity, and metabolic syndrome in adults and children in South Korea: a review of the literature. Clin. Nurs. Res 20, 276–291 (2011).
Hou, X. et al. Impact of waist circumference and body mass index on risk of cardiometabolic disorder and cardiovascular disease in Chinese adults: a national diabetes and metabolic disorders survey. PLoS ONE 8, e57319 (2013).
Wang, Y. et al. Prevalence and numbers of diabetes patients with elevated BMI in China: evidence from a nationally representative cross-sectional study. Int J. Environ. Res. Public Health 19, 2989 (2022).
Ji, L. N. & Zou, D. J. Consensus of chinese experts on the remission of type 2 diabetes mellitus. Chin. Gen. Pract. 24, 11 (2021).
Heise, T. et al. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care 46, 998–1004 (2023).
Francula-Zaninovic, S. & Nola, I. A. Management of measurable variable cardiovascular disease’ risk factors. Curr. Cardiol. Rev. 14, 153–163 (2018).
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018).
Aroda, V. R. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes. Metab. 20, 22–33 (2018).
Technical Guidance for Clinical Trials of Drugs for the Treatment of Adults with Type 2 Diabetes (China National Medical Products Administration, 2023); https://www.cde.org.cn/main/att/download/e2cb472cc84a13e946378f819cc5c206
Acknowledgements
This study was funded by Eli Lilly and Company. The study sponsor had a role in the study design, data collection, data analysis, interpretation of data, writing of the report and in the decision to submit the paper for publication. Medical writing assistance in the preparation of this article was provided by M. Dyson on behalf of Rude Health Consulting, J. Burrell (Rude Health Consulting) and C. Zeng (Eli Lilly and Company) and was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
L.J. led the design, conduct and analysis of the clinical study. L.G., B.L., M.C. and J.K. contributed to the acquisition of data. L.D. led the data analysis, and all authors participated in data interpretation. All authors confirm that they had full access to the complete study data, took part in the development of the manuscript and accept responsibility to submit for publication.
Corresponding author
Ethics declarations
Competing interests
L.H., L.D. and Y.H. are employees of Eli Lilly and Company. L.J. reports receiving consulting and lecture fees from Eli Lilly and Company, Novo Nordisk, Merck, Bayer, Sanofi-Aventis, Roche, Merck Sharp & Dohme, Metronics, AstraZeneca, Boehinger Ingelheim and Abbott. L.J. and L.G. received a research grant for this study from Eli Lilly and Company. M.C. reports having speaker contracts with Novo Nordisk, Sanofi, Biocon, Cipla, USV Ltd. and Abbott. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Victor Volovici, Stefano Del Prato and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Change from baseline in HbA1c (a) and proportion of patients achieving HbA1c < 7.0% (b) at week 40 in the full analysis set.
LSMean changes from baseline in HbA1c were estimated using an ANCOVA with return-to-baseline multiple imputation for missing values at week 40 in patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); statistical tests for 10 mg and 15 mg were two sided at a significance level of 0.025, statistical tests for 5 mg were two sided at a significance level of 0.05. Proportion of patients achieving HbA1c < 7.0% was estimated using logistic regression analysis with return-to-baseline multiple imputation for missing values at week 40 in patients who received at least 1 dose of study drug (5 mg, n = 230; 10 mg, n = 228; 15 mg, n = 229; insulin glargine, n = 220); all statistical tests were two sided at a significance level of 0.05. Error bars indicate SE. ANCOVA, analysis of covariance; HbA1c, glycated hemoglobin; LSMean, least-square mean; n, number of patients achieving target with missing value imputed by return-to-baseline multiple imputation; N, number of patients who were randomized and received at least 1 dose of study drug; SE, standard error.
Extended Data Fig. 2 Change from baseline in bodyweight at week 40 in the full analysis set.
Changes from baseline in bodyweight were estimated using an ANCOVA with return-to-baseline multiple imputation for missing values at week 40 in patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); statistical tests for 10 mg and 15 mg were two sided at a significance level of 0.025, statistical tests for 5 mg were two sided at a significance level of 0.05. Data presented are LSMean (SE). Error bars indicate SE. ANCOVA, analysis of covariance; LSMean, least-square mean; N, number of patients who were randomized and received at least 1 dose of study drug; SE, standard error.
Extended Data Fig. 3 Incidence and severity of nausea, vomiting, diarrhea and decreased appetite over time.
Notes: dotted areas indicate the tirzepatide dose escalation period. Severity is indicated by color: green (mild); orange (moderate); red (severe). TZP, tirzepatide.
Extended Data Fig. 4 P-amylase (a) and lipase (b) levels over time.
Data presented are estimate mean (SE). Error bars indicate SE. Baseline values were calculated using an ANOVA, post-baseline measures were calculated from an MMRM, all statistical tests were two sided at a significance level of 0.05. Normal reference values: p-amylase (13–53 IU/L); lipase (13–60 IU/L). ANOVA, analysis of variance; MMRM, mixed model for repeated measures; No., number of patients with baseline and post-baseline value at the specified time point; p-amylase, pancreatic amylase; TZP, tirzepatide; SE, standard error; SFU, safety follow up.
Extended Data Fig. 5 Change in waist circumference over time.
Data presented are LSMean (SE). Error bars indicate SE. LSMean, least-square mean; No., number of patients with baseline and post-baseline value at the specified time point; SE, standard error.
Extended Data Fig. 6 Percent changes in ALT (a) and AST (b) over time.
Data presented are estimate mean (SE). Error bars indicate SE. Baseline values were calculated using an ANOVA, post-baseline measures were calculated from an MMRM, all statistical tests were two sided at a significance level of 0.05. ANOVA, analysis of variance; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MMRM, mixed model for repeated measures; No., number of patients with baseline and post-baseline value at the specified time point; TZP, tirzepatide; SE, standard error; SFU, safety follow up.
Extended Data Fig. 7 Subgroup analysis of change in HbA1c from baseline to week 40 according to baseline oral anti-hyperglycemic medication use and enrolment in China versus out of China.
ETD and CIs in HbA1c at week 40 were estimated using an MMRM without missing-value imputation in the patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); all statistical tests were two sided at a significance level of 0.05 and no adjustments were made for multiplicity. Data presented are LSMean. Error bars indicate 95% CI. P values represent treatment-by-subgroup interaction at week 40. ETD, estimated treatment difference; CI, confidence interval; CHN, China; HbA1c, glycated hemoglobin; LSMean, least-square mean; MMRM, mixed model for repeated measures; N, number of patients who were randomized and received at least 1 dose of study drug; OAM, oral anti-hyperglycemic medication; OCHN, out of China; SU, sulphonylurea; TZP, tirzepatide.
Extended Data Fig. 8 Subgroup analysis of change in bodyweight from baseline to week 40 according to baseline oral anti-hyperglycemic medication use and enrolment in China versus out of China.
ETD and CIs in bodyweight at week 40 were estimated using an MMRM without missing-value imputation in the in patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); all statistical tests were two sided at a significance level of 0.05 and no adjustments were made for multiplicity. Data presented are LSMean. Error bars indicate 95% CI. P values represent treatment-by-subgroup interaction at week 40. ETD, estimated treatment difference; CI, confidence interval; CHN, China; LSMean, least-square mean; MMRM, mixed model for repeated measures; N, number of patients who were randomized and received at least 1 dose of study drug; OAM, oral anti-hyperglycemic medication; OCHN, out of China; SU, sulphonylurea; TZP, tirzepatide.
Extended Data Fig. 9 SURPASS-AP-Combo trial design.
FBG, fasting blood glucose; QW, once weekly; QD, once daily; SU, sulfonylurea. a Stable doses of metformin (metformin ≥1000 mg/day and no more than the maximum approved dose per country-specific label) and/or a sulfonylurea for 2 months prior to Visit 1, and during the screening/lead-in Period. b The initial dose of insulin glargine was 6 IU/day for patients who had an average FBG concentration of ≥7.8 mmol/L (140 mg/dL). The initial dose of insulin glargine for patients with an average FBG concentration of <7.8 mmol/L (<140 mg/dL) might be reduced by 1-2 IU/day at the investigator’s discretion. Note: Patients titrated insulin glargine dose in a weekly manner and made the dose decision with the investigator for the first 8 weeks (phone or clinic visit). From week 8 to week 16 patients continued the titration by a phone consultation or clinic visit every other week. It was expected that the insulin dose stayed relatively stable from week 16 onwards.
Extended Data Fig. 10 Graphical multiple-testing procedure for the primary and key secondary efficacy endpoints.
HbA1c, glycated hemoglobin; TZP, tirzepatide.
Supplementary information
Supplementary Information
The file contains Supplementary Tables 1–10; criteria for severe, persistent hyperglycemia; and a complete list of eligibility criteria from the protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, L., Lee, B.W., Chawla, M. et al. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: the SURPASS-AP-Combo trial. Nat Med 29, 1500–1510 (2023). https://doi.org/10.1038/s41591-023-02344-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02344-1
This article is cited by
-
Comparative Efficacy and Safety of Tirzepatide in Asians and Non-Asians with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Diabetes Therapy (2024)
-
Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes: Analysis of SURPASS-AP-Combo by Different Subgroups
Diabetes Therapy (2024)
-
Evaluating the effectiveness and safety of various Tirzepatide dosages in the management of Type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials
Journal of Diabetes & Metabolic Disorders (2024)
-
Tirzepatide: A Review in Type 2 Diabetes
Drugs (2024)
-
Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials
Diabetologia (2024)