Abstract
Intermittent fasting appears an equivalent alternative to calorie restriction (CR) to improve health in humans. However, few trials have considered applying meal timing during the ‘fasting’ day, which may be a limitation. We developed a novel intermittent fasting plus early time-restricted eating (iTRE) approach. Adults (N = 209, 58 ± 10 years, 34.8 ± 4.7 kg m−2) at increased risk of developing type 2 diabetes were randomized to one of three groups (2:2:1): iTRE (30% energy requirements between 0800 and 1200 hours and followed by a 20-h fasting period on three nonconsecutive days per week, and ad libitum eating on other days); CR (70% of energy requirements daily, without time prescription); or standard care (weight loss booklet). This open-label, parallel group, three-arm randomized controlled trial provided nutritional support to participants in the iTRE and CR arms for 6 months, with an additional 12-month follow-up. The primary outcome was change in glucose area under the curve in response to a mixed-meal tolerance test at month 6 in iTRE versus CR. Glucose tolerance was improved to a greater extent in iTRE compared with CR (−10.10 (95% confidence interval −14.08, −6.11) versus −3.57 (95% confidence interval −7.72, 0.57) mg dl−1 min−1; P = 0.03) at month 6, but these differences were lost at month 18. Adverse events were transient and generally mild. Reports of fatigue were higher in iTRE versus CR and standard care, whereas reports of constipation and headache were higher in iTRE and CR versus standard care. In conclusion, incorporating advice for meal timing with prolonged fasting led to greater improvements in postprandial glucose metabolism in adults at increased risk of developing type 2 diabetes. ClinicalTrials.gov identifier NCT03689608.
Similar content being viewed by others
Main
Dietary interventions that involve moderate calorie restriction (CR) are an established strategy for weight management and reduce the risk of developing type 2 diabetes for over 10 years1,2. However, an emerging area of research is the role of meal timing and prolonged fasting in extending the health benefits of CR.
Intermittent fasting (IF), defined as a period of fasting interspersed with days of ad libitum eating, has gained popularity as an alternative to CR3. An umbrella review of meta-analyses showed that IF is an equivalent alternative to CR for weight loss and health outcomes assessed in the fasting state4. Only two trials have been powered to assess insulin sensitivity by homeostatic model assessment for insulin resistance5,6, reporting greater improvements in IF versus CR. To our knowledge, no studies have been powered for postprandial assessments of glycemia, which are better indicators of diabetes risk than fasting assessment. Furthermore, it is theorized that IF may provide a greater stimulus than CR for altering nutrient signaling pathways, lipid metabolism and insulin sensitivity7. Thus, high-quality evidence on whether IF is an effective strategy to improve glucose tolerance compared with CR is lacking in humans. Furthermore, the comparative effects of IF and CR on β-hexosaminidase, a lysosomal isozyme involved in the degradation of glycosaminoglycans, gangliosides and oligosaccharides8, have not been tested. Plasma β-hexosaminidase activity is elevated in people who live with diabetes and/or Alzheimer’s disease9—both age-related diseases with shared behavioral risk factors—and is a clinical marker of liver health10,11,12.
Most IF protocols have prescribed ~30% of energy needs on two to three (consecutive or nonconsecutive) ‘fasting’ days per week, but have not specified when food is eaten during the ‘fasting’ day13. This is a limitation because it has become clear that provision of food at the onset of the active phase with prolonged fasting during the rest phase maximized the longevity and health benefit of CR in mice14,15,16. Alongside this evidence, time-restricted eating (TRE) has emerged as a novel form of IF that emphasizes a daily shorter eating window (4–10 h) in alignment with circadian rhythms17. Three randomized controlled trials have compared the benefits of adding TRE with a CR diet in adults with obesity18,19,20. TRE did not add to CR-induced improvements in body composition, blood lipids or glucose parameters18,19, although one study showed 1.5% greater weight loss and greater reductions in blood pressure after 14 weeks with early TRE (limiting food intake to between 0700 and 1500 hours daily)20. In these studies, the fasting periods were 14–16 h, which may be insufficient to activate some metabolic pathways21.
Here, we tested IF plus early time-restricted eating (iTRE), by allowing 30% energy requirement to be consumed before 1200 hours, followed by a 20-h fasting period on three nonconsecutive days per week compared with CR and a standard care group in adults at elevated risk of developing type 2 diabetes.
Results
Trial participants
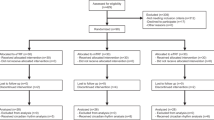
In total, 209 participants (57% female, 58 ± 10 years, 34.8 ± 4.7 kg m−2) were enrolled between 26 September 2018 and 4 May 2020: 85 (40.7%) were allocated to iTRE, 83 (39.7%) were allocated to CR and 41 (19.6%) were allocated to standard care (Fig. 1 and Table 1). At month 6, 46 participants had withdrawn: 29 did not wish to continue, 12 were lost to contact, 4 withdrew owing to health conditions unrelated to the study and 1 was withdrawn by the investigators owing to very low blood pressure at baseline.
Glycemic control
There was a greater improvement in the primary outcome postprandial glucose area under the curve (AUC) at month 6 in iTRE (−10.10 (95% confidence intervals (CI) −14.08, −6.11) mg dl−1min−1) versus CR (−3.57 (95% CI −7.72, 0.57) mg dl−1 min−1) (P = 0.03; Table 2 and Fig. 2a). In a secondary analysis, the comparison of glucose AUC at month 6 across all groups did not reach statistical significance (P = 0.06). The reduction in postprandial insulin AUC was greater in iTRE versus CR and standard care at month 6 (Table 2 and Fig. 2b). Glycated hemoglobin (HbA1c) was reduced in both iTRE and CR versus standard care at month 6. No between-group differences were detected in the reduction in fasting glucose or insulin (Table 2), or postprandial nonesterified fatty acids (NEFA) AUC (Fig. 2c). However, a greater reduction was observed in fasting NEFA in iTRE versus CR at months 2 and 6, and versus standard care at month 6 (Table 2). There were no qualitative differences between the frequentist and Bayesian secondary analyses of postprandial glucose and HbA1c change (Supplementary Table 1 and Extended Data Fig. 1).
a–c, Mean glucose (a), insulin (b) and NEFA (c) in iTRE and CR conducted during baseline (iTRE n = 81; CR n = 78) and month 6 (iTRE glucose n = 67, insulin and NEFA n = 64; CR glucose, insulin and NEFA n = 62). Presented are means and 95% CI (calculated as \(\overline x \pm 1.96\,{{{\mathrm{s.e.m.}}}}\)). AUC values were compared between iTRE and CR at 6 months adjusted for sex, AUSDRISK and baseline values. P values are two-sided and not adjusted for multiple testing.
Cardiovascular risk
There were greater reductions in systolic blood pressure in iTRE and CR versus standard care at month 2, and CR versus standard care only at month 6 (Table 2). A greater reduction in diastolic blood pressure also occurred in both iTRE and CR versus standard care at month 6. Fasting triglycerides were lower in iTRE versus CR and standard care at month 2 and 6. There were no between-group differences detected in postprandial triglycerides. Total cholesterol and low-density lipoprotein (LDL) were lower in iTRE versus standard care at month 6. A greater reduction in the cholesterol to HDL ratio was observed in iTRE versus CR at month 2, and versus standard care at months 2 and 6.
Liver health
There was no significant difference between groups in the reduction in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) at month 6 (Table 2). There was a greater reduction in plasma β-hexosaminidase activity in iTRE (−24.70 (95% CI −33.81, −15.59) pmol min−1 mg−1) versus CR (−8.91 (95% CI −18.32, 0.50) pmol min−1 mg−1) and versus standard care (4.51 (95% CI −8.51, 17.53) pmol min−1 mg−1) at month 6 (Table 2).
Body weight and composition
Body weight and fat mass loss were greater in both iTRE and CR versus standard care over the first 6 months (Table 3). There were no detectable differences in weight trajectories between the iTRE and CR groups (Supplementary Table 2 and Extended Data Figs. 2 and 3). Waist circumference was reduced in iTRE and CR versus standard care at month 6 (Table 3).
Physical activity and self-reported dietary intake
Physical activity did not differ between groups (Supplementary Table 3). However, self-reported energy intake was modestly lower in iTRE versus CR at month 2. Protein and fiber intakes were also lower at months 2 and 6 in iTRE versus CR. Energy intake during iTRE and ad libitum days at month 2 did not differ from month 6, indicating comparable adherence over time in iTRE (Supplementary Table 4).
Follow-up data at 18 months
When participants were offered the choice to modify their diet at month 6, 32 of 69 (46%) of iTRE said they would maintain 3 days of iTRE per week, 35 of 69 (51%) chose to reduce to 2 days of iTRE per week, 1 said they would follow 1 day of iTRE per week, and 1 indicated they would no longer follow iTRE. By contrast, 60 of 62 (97%) of the CR completers indicated they would continue their current diet plan, and 2 chose to modify to a weight maintenance plan. At month 18, 23 of 55 (42%) iTRE completers reported they undertook 2–3 days of iTRE per week, whereas 42 of 54 (78%) CR completers reported they followed a CR diet. However, there was no difference in weight or body composition between groups at month 18 (Extended Data Fig. 2 and Table 3). There were also no between-group differences in the reported biomarkers of health (Table 2), except for lower waist circumference in CR versus standard care.
Exploratory analyses
We calculated insulin sensitivity using the Matsuda index and insulin secretion using the insulinogenic index, neither reached statistical significance (Table 2). We divided iTRE into those who indicated they would continue with their initial plan (3 days of iTRE per week), and those who chose to reduce the number of iTRE days per week, but we did not detect any between-group differences (Supplementary Table 5). We also combined iTRE with CR and observed lower insulin AUC, and a trend for differences in glucose and NEFA AUC versus standard care (Supplementary Table 6).
Safety outcomes
In the first 6 months, 56 of 209 participants reported at least one adverse event, with flu-like symptoms most frequently reported, and there was no difference in the incidence between groups (Table 4). During the follow-up period, 65 of 134 participants reported at least one adverse event, with back pain and flu-like symptoms most common, and there was a higher incidence in iTRE (45%) and CR (45%) versus standard care (19%). Four serious adverse events with hospitalization were reported, one in first 6 months and three during follow-up; none were considered to be related to the intervention. Fatigue was higher in iTRE (56%) versus CR (37%) and standard care (35%) over the first 6 months and during follow-up. Constipation was higher in iTRE (33%) and CR (27%) versus standard care (5%) over the first 6 months, and headaches were higher in iTRE (26%) and CR (26%) versus standard care (6%) during follow-up.
Discussion
This randomized controlled trial demonstrated that iTRE was superior to CR for improving postprandial glucose tolerance in adults at increased risk of developing type 2 diabetes.
Our findings are in alignment with past studies of IF versus CR showing similar effectiveness for body weight, fat mass, fasting glucose and insulin22,23. However, postprandial responses to a mixed-nutrient meal are a better assessment of glycemic control than simple fasting assessments, more highly predictive of type 2 diabetes and cardiovascular disease, and provide more physiological relevance than oral glucose tolerance tests24,25,26,27. In this large trial, superior improvements in glucose tolerance were observed in iTRE versus CR after 6 months, independently of body weight loss. Only three pilot studies have previously compared the effects of IF versus CR on postprandial glucose and lipid metabolism in humans. One showed a greater reduction in postprandial triglycerides following IF, but there were no differences in postprandial glucose or insulin in individuals with obesity (n = 16 per group)28 or without obesity (n = 8–12 per group) after 2–8 weeks29,30. Improvements in glucose tolerance can occur through improved insulin sensitivity, insulin secretion, glucose mass action or slower gastric emptying. We observed a greater reduction in postprandial insulin AUC, which could indicate greater improvements in insulin sensitivity. Early TRE (0800 to 1600 hours) was previously shown to improve glucose tolerance by increasing insulin sensitivity and the efficiency of glucose extraction in skeletal muscle after 2 weeks in healthy men31. In the current study, greater reductions in fasting NEFA were observed, which suggest greater improvements in adipose tissue insulin sensitivity, which could also reduce ectopic lipid and increase peripheral glucose uptake32. There was no difference in insulin secretion as calculated by the insulinogenic index33. Gastric emptying was not assessed, but could also have partly contributed to the improvement in glucose tolerance34, although gastric emptying was not changed following early TRE (0800 to 1700 hours) after 1 week35. The greater improvements in glucose tolerance did not result in a greater reduction in HbA1c. HbA1c is influenced by both postprandial and fasting glucose, although postprandial glucose is most strongly predictive of HbA1c in adults without diabetes. Although HbA1c was marginally elevated, it is minimally shifted with dietary interventions in those without type 2 diabetes36.
Few studies have specified morning meal timing during IF in humans13, potentially masking its possible health benefits. Eating at an early time in the day is associated with health benefits in humans in short-term trials37, including improved 24-h glucose control38 and insulin sensitivity in response to an oral glucose tolerance test39 and glucose mass action31. Adding early TRE (0700 to 1500 hours) to a CR diet was also effective for weight loss and reducing diastolic blood pressure after 14 weeks20. Delaying food intake is associated with reduced weight loss in retrospective cohort studies37 and increased hunger and reduced energy expenditure in an acute metabolic ward study40. Delayed food intake also delayed adipose tissue clocks and expression of Period-2 in humans41. Thus, although a delayed eating plan may be more socially acceptable and sustainable, it may not be optimal for glycemic health22,23. In contrast to previous long-term IF trials23,29, we provide preliminary evidence that prescription of iTRE elicits favorable alterations in lipid profiles versus CR, including reductions in fasting triglycerides, which are strongly associated with an increased risk of cardiovascular diseases42,43. Fatty liver is also commonly associated with increased very-LDL triglycerides secretion and hypertriglyceridemia, and thus might also reflect an increased reduction in liver fat32. Several studies have shown increased β-hexosaminidase activity in both diabetes and liver disease10,11,44, and one study has extended these findings to Alzheimer’s disease9. Interventions that improve glycemic control reduce β-hexosaminidase activity45,46. Here, iTRE was more effective in reducing β-hexosaminidase activity. This exploratory finding could also reflect superior restoration of lipid metabolism12,47 and liver health compared with CR, but the clinical relevance of a change in β-hexosaminidase with a dietary intervention is uncertain.
Adverse events were generally mild and resolved over the course of the trial. As iTRE and CR may increase feelings of fatigue, light-headedness, constipation and/or headache, we prompted participants to report on these physical symptoms. Higher fatigue was observed in iTRE versus CR during the first 6 months, which might increase difficulty in maintaining iTRE. Fasting protocols that require people to skip dinner several times per week could also lead to poor adherence. Fewer than half of the iTRE participants indicated they would continue their current weight loss plan when they were given the option to modify to a weight maintenance plan that included two iTRE days per week at month 6. By contrast, 97% of CR participants said they would continue their current weight loss plan. However, weight loss maintenance at month 18 did not differ between iTRE and CR, suggesting that neither regimen was more sustainable when support from the investigators was withdrawn. The increased health benefits in the active intervention groups were also lost by 18 months. This was partly due to a loss in power with 35% withdrawal. A post hoc subanalysis that divided the iTRE cohort into those that chose to continue versus change the diet plan did not alter outcomes. As an exploratory analysis, we also combined iTRE and CR, and observed sustained reductions in insulin AUC versus standard care. Future studies should investigate whether intermittent prescription of a longer daily eating window (for example, 0800 to 1600 hours) retains the benefit of iTRE versus CR, and if these interventions are more sustainable long-term.
Strengths of the study include that it is the largest to date, with a high proportion of men enrolled. This trial also included assessments of glucose tolerance and aligned meal timing from breakfast during IF. The limitations include utilizing 2:2:1 randomization, which affected the ability to detect differences between ‘active’ and ‘inactive’ groups, particularly at the 18-month follow-up. In addition, some individuals in the standard care group were highly self-motivated, with 8 of 41 experiencing clinically relevant weight loss (>5%), which could yield smaller between-group differences in metabolic health-related outcomes48. At month 6, we allowed participants in the active groups to choose whether they changed to a weight maintenance plan during the additional 12-month follow-up. This trial decision has hindered the clinical interpretation of that period because more participants in the iTRE group chose to alter their diet plans. Whether 24-h post refeeding is sufficient to entirely wash out the acute effect of the 20-h fasting period is not clear, although the washout period is in line with past studies of IF29. CR and standard care participants were not provided with any instruction on meal timing, and adoption of a shortened daily eating period might have lessened the differences between groups. The extrapolation of a clinically relevant change in glucose from an oral glucose tolerance test to a mixed-meal tolerance test requires further study. Finally, although we theorized that limiting meals to the morning during iTRE was responsible for the greater health benefits that were observed versus CR, we did not include an iTRE group with a late eating window as a comparator.
In conclusion, iTRE provided modest benefit for postprandial glycemia in response to mixed-meal tolerance test compared with daily CR without timing advice in adults at elevated risk of type 2 diabetes after 6 months. This study adds to the growing body of evidence to indicate that meal timing and fasting advice might be influential in clinical practice.
Methods
Study design and participants
This open-label, three-arm, parallel group sequential randomized controlled trial was conducted between 26 September 2018 and 30 November 2021 and involved a 6-month intervention phase followed by a 12-month follow-up. The primary objective for this study was to assess differences in glucose tolerance in response to a mixed-meal in iTRE versus CR at 6 months. Because it was expected that weight loss for iTRE and CR would be similar, a standard care group was included to ensure weight losses occurred and to aid quantification of the magnitude of change in the active intervention groups. Secondary aims were to compare iTRE versus CR versus standard care on body weight, body composition, fasting and postprandial markers of glycemia, cardiovascular health and liver health at 6 months, and with a further 12-month follow-up in adults at elevated risk of developing type 2 diabetes. Eligible participants (aged 35–75 years, score ≥12 on the Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK), weight fluctuations ≤5% for more than 6 months before study entry, without a diagnosis of diabetes, not taking medications that might affect glucose metabolism and/or weight management) were randomized to either iTRE or CR or standard care (2:2:1 ratio). Randomization was block-stratified by sex (male; female) and AUSDRISK (score ≥12 to 19; score ≥20), according to a computer-generated randomization list prepared by a statistician with no clinical involvement in the trial. The detailed study protocol including inclusion and exclusion criteria was reported49,50, and the statistical analysis plan is available (ClinicalTrials.gov, NCT03689608). Ethics approval was obtained from the Central Adelaide Local Health Network Human Research Ethics Committee and participants provided written informed consent. The study was performed at the South Australian Health and Medical Research Institute by researchers from The University of Adelaide and South Australian Health and Medical Research Institute. An independent data and safety monitoring committee provided oversight. In response to the coronavirus pandemic, a lockdown was in place in South Australia from mid-March to May 2020, which brought a halt to recruiting. The primary outcome visits continued, but the diet consults were shifted from face-to-face to telehealth. Other than this period, the Australian border force laws in place meant the study visits remained largely unaffected, with the final follow-up visit completing around the time that Adelaide relaxed its border rules.
Diet interventions
The iTRE group was instructed to consume 30% of calculated baseline energy requirements51 as per a prescribed menu on fasting days, followed by a 20-h fast from 1200 hours on three nonconsecutive days per week. The prescribed menu included two meal replacements at breakfast (approximately 0800 hours) and lunch (approximately 1200 hours) to aid adherence and to ensure adequate nutrient intake. iTRE participants were instructed to consume their regular prestudy diet during each nonfasting day. The CR group was prescribed 30% restriction of calculated baseline energy requirements51 daily and given rotating menu plans that included a dinner meal and snacks, but were not specifically instructed on meal timing. The prescribed menu included one meal replacement per day to aid adherence and to ensure adequate nutrient intake. The standard care group was given current guidelines in a booklet, with no counseling or meal replacements. All groups underwent identical clinic visits fortnightly during the first 6 months and every other month during the 12-month follow-up. All participants were instructed to maintain their usual physical activity levels throughout the trial. Only the iTRE and CR groups were provided one-on-one diet counseling during first 6 months. At month 6, they were provided with the option to continue with the same weight loss plan or to modify to a weight maintenance plan. The modified plan for iTRE was to reduce the number of iTRE days to once or twice per week and CR were given new target calories that increased by 10%–15% above their current plan.
Outcome measures
The primary outcome was the change in postprandial glucose AUC at 6 months. The secondary outcomes included changes in body weight, waist circumference, hip circumference, fat mass, fat-free mass, blood pressure, blood lipids (cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), plasma triglycerides), NEFA, HbA1c, plasma glucose, plasma insulin, serum high-sensitivity C-reactive protein (hs-CRP), ALT, AST, β-hexosaminidase activity, physical activity and dietary intake.
Body composition
During each metabolic visit, body weight, and waist and hip circumference were measured in a gown after voiding. Body weight was measured to the nearest 0.1 kg with a calibrated scale (Tanita BWB-800 digital scale, Wedderburn). Waist circumference was measured at the mid-axillary line (halfway point between lowest rib and the top of iliac crest), and hip circumference was measured at the widest circumference of the buttocks. Body mass index was calculated as weight in kilograms per height in meters squared. Whole-body composition was measured by dual-energy X-ray absorptiometry (DXA) (Lunar Prodigy; GE Health Care) and was analyzed using enCORE software (v.18). Owing to the coronavirus pandemic, the DXA facility was shut for 5 months, thus there were more missing DXA data during the first 6 months (see available N in Supplementary Table 7). We calculated the ratio of fat mass to weight loss, and the ratio of fat-free mass to weight loss, which was defined as the change in fat mass or fat-free mass respectively, divided by the change in total body weight × 100. These were assessed in completers who lost at least 3.6 kg to account for the potential errors by DXA20.
Blood pressure
Blood pressure was measured twice with the participant seated after 10 min of rest (Welch Allyn Medical Products). A third measurement was taken if there was a difference of >3 mmHg. The mean of the two lowest blood pressure readings was used.
Dietary intake
Prescribed daily energy requirements were calculated by averaging predicted daily energy expenditure from a published equation that uses gender, age, height and weight variables51. Participants were asked to self-report all their dietary intake via a smartphone application (Easy Diet Diary, Xyris Software) before each metabolic testing at baseline, and at months 2, 6 and 18. Adherence to the diets was assessed by dietitians using the participants’ self-reported 7-day food records. The energy and macronutrients intakes were calculated by using FoodWorks Professional v.10 (Xyris Software). Perceptions of diet easiness and satisfaction were assessed at months 2 and 6 using visual analog scales.
Physical activity
Participants were fitted with a waist-worn triaxial accelerometer (ActiGraph wGT3X-BT, LLC) secured above the right hip bone using an elastic belt for measuring human movements and activities for at least 7 days before each metabolic testing. The monitor was initialized at a sample rate of 30 Hz to record activities for free-living conditions. ActiGraph data was downloaded and analyzed by using ActiLife 6 software by the investigators upon collection of the devices.
Meal tests
Participants attended the research facility at baseline, month 6 and month 18 for metabolic testing. Participants arrived at 0730 hours after an overnight 12-h fast, fasting samples (t = 0) were drawn, and participants were then asked to consume a mixed-nutrient liquid test meal (474 ml Nestle Resource Plus, 730 kcal: 59.4% carbohydrate, 14.2% protein, 26.4% fat) within 5 min. Subsequent postprandial blood samples were drawn at 15, 30, 60, 90, 120 and 180 min. Additional fasting samples were obtained following a 12-h fast at month 2. The blood collection procedure was standardized, with participants in the iTRE group attending clinic 24 h after the completing their previous 20-h fasting period.
Blood analysis
Blood glucose was assayed by the hexokinase method (Cobas Integra 400 plus, Roche). Plasma insulin was measured by radioimmunoassay (HI-14K, Millipore). Whole-blood HbA1c, plasma triglycerides, NEFA, hs-CRP, ALT and AST, were measured using commercially available enzymatic kits on an automated clinical analyzer (Indiko Plus, Thermo Fisher Scientific). AUC values were calculated using the trapezoidal rule. The Matsuda index was calculated for insulin sensitivity estimation52. Insulin secretion was estimated using the insulinogenic index33.
A subset of 121 individuals had additional fasting bloods drawn at baseline, month 2 and month 6 to assess plasma β-hexosaminidase activity as a marker of glycosphingolipid metabolism relevant to liver health. β-Hexosaminidase activity was measured using a plasma sample as described in Leaback et al. and Whyte et al.53,54. Plasma samples were thawed on ice, vortexed and diluted 1:5 in ice-cold 0.9% saline. Diluted samples (5 µl) were loaded in triplicate in a 96-well plate maintained on ice. Saline solution (0.9%) was used as a blank. 4-Methylumbelliferyl-N-acetyl-β-d-glycosaminide (Sigma, catalog no. 69585; β-hexosaminidase substrate) was prepared by dissolving 4.7 mg of β-hexosaminidase substrate in 10 ml of citrate-phosphate buffer (pH 4.8, final concentration of 1.24 nmol l−1). Then 100 µl of β-hexosaminidase substrate was added to each well. The plate was incubated for 30 min at 37 °C. The reaction was stopped by adding 100 µl of glycine buffer (0.2 mol l−1, pH 10.7). 4-Methylumbelliferone was used as standard (Sigma, catalog no. M1381; 2.84 nmol). Fluorescence was read on a GloMax microplate reader (Promega). Results are reported as pmol min−1 mg−1 and calculated by comparing the average fluorescence readings of each sample with the average of the known standard as such: β-hexosaminidase activity (pmol min−1 mg−1) = (fluorescence units (FU) sample − FU blank)/FU standard × standard amount (2,840 pmol)/incubation time (30 min)/volume of sample loaded (5 µl) × dilution factor (5) × 1,000/plasma protein concentration (mg ml−1).
Safety outcomes
During each clinic visit, participants were asked to report if they had experienced any health-related conditions. They were also prompted to report any physical symptoms through the use of a check box (for example, fatigue, constipation, diarrhea, headache, light-headedness) since the proceeding visit. All serious adverse events were immediately reported to the study physician and data safety monitoring committee. For each assessment period (baseline to month 6, and month 7 to month 18), the number of individuals with at least one event was compared between groups when there were at least four individuals with at least one event across all groups. Fisher’s exact test was conducted to test whether there were any differences between groups.
Sample size considerations
The original study design was a group sequential design (interim times at 1/6, 1/3 and 1/2) with dual primary outcomes (HbA1c and postprandial glucose AUC). The design was changed after the first interim analysis to a single additional (final) analysis of postprandial glucose AUC owing to slow accrual and the coronavirus pandemic. This change was agreed by the independent data safety monitoring committee2. We assume a pre–post intervention correlation of 0.4, within-group s.d. of 0.8, and an anticipated attrition percentage of 25%. Thereby, with n = 208 (randomized iTRE to CR to standard care = 2:2:1) there would be >85% power to detect a clinically relevant36 difference of 7.2 mg dl−1 min−1 in the change of postprandial glucose AUC between iTRE and CR at 6 months (overall two-sided α = 0.05). According to the original design, only a very small alpha (<1 × 10−6) was spent at the interim analysis, thereby the significance of primary analysis was set at P < 0.049999.
Statistical methods
Baseline data are presented as mean ± s.d. for continuous measures and n (%) for discrete measures. The primary analysis of month 6 postprandial glucose AUC between iTRE and CR was assessed using baseline and stratification factor (sex, AUSDRISK) adjusted linear regression. Other analyses also included the standard care and where appropriate the month 2 assessment. The latter were modeled using mixed effects linear regressions with a random intercept per individual and adjusted for assessment (month 2 versus month 6) and the pairwise interaction with treatment group as fixed effects. Residual and random effect distributions were assessed to ensure that the model distributional assumptions were not violated. Fasting triglycerides, hs-CRP, AST, ALT, Matsuda index, insulinogenic index and step counts outcomes were log-transformed. Estimates and 95% CI values are calculated on the log-transformed scale and reported on the original scale using following formula \(\left( {e^{\widehat{\beta} } - 1} \right)\overline x _0\), where \({\widehat{\beta}}\) is the estimate or interval limit and \(\overline x _0\) is the baseline sample mean. With three groups and two assessment times there are a number of potential secondary outcome comparisons. We prespecified that pairwise comparisons of secondary analyses would be performed only if the overall effect of treatment group was significant in a likelihood ratio test with the nested submodel excluding treatment. For these overall tests, mixed effects models did not include the month by group interaction (that is, the likelihood ratio test statistic was compared against the chi-squared distribution with two degrees of freedom for all outcomes irrespective of the month 2 assessment). Month 18 assessments were analyzed separately using linear regressions similarly to secondary outcomes without a month 2 assessment. A post hoc analysis was performed repeating these regressions in which the iTRE group was divided into those who chose to maintain the initial iTRE weight loss plan and those who chose to modify to a weight maintenance plan. Nonfasting weight assessments were analyzed using linear mixed effects regression assuming piecewise linear effects assumed for the interventions over two periods: months 0–6 and months 7–18, and both random intercepts and slopes for individuals. In all analyses, missing baseline data were imputed using cohort means, estimated means and 95% CI values (as mean (95% CI) units, P value) are reported, and the threshold for significance was set at 0.05 (two-sided). No multiple test adjustments were performed and as such secondary analyses are considered exploratory. Statistical analysis was performed using R v.4.0.5 (The R Foundation for Statistical Computing).
Secondary Bayesian joint analysis of postprandial glucose and HbA1c
We prespecified a skeptical bivariate normally distributed prior with mean δ0 = 0 for both HbA1c and postprandial glucose. Using a historical series of clinical trials in diabetes patients the prespecified covariance matrix was \({{{\Sigma}}}_0 = \left[ {\begin{array}{*{20}{c}} {0.14} & {0.081} \\ {0.081} & {0.096} \end{array}} \right],\)corresponding to a between-study variance for HbA1c (%) of 0.14 and 0.096 for postprandial glucose (mmol l−1 min−1), and a 0.7 correlation in outcomes across studies. Converting glucose units to mg dl−1 this prior covariance becomes \({{{\Sigma}}}_0 = \left[ {\begin{array}{*{20}{c}} {0.14} & {1.46} \\ {1.46} & {31.2} \end{array}} \right]\).
Using the standard assumption, the observed covariance \({{{\mathrm{{\Sigma}}}}}_{{{{\mathrm{{\Delta}}}}}\overline {{{{y}}}} }\) is known, the posterior distribution is also bivariate normally distributed with posterior mean
and posterior covariance
where \({{{\mathrm{{\Delta}}}}}\overline {{{{y}}}}\) is the mean difference between groups in the change from baseline HbA1c and postprandial glucose. We prespecified when reporting posterior probabilities that the difference between groups in HbA1c is >0.3% and that postprandial glucose is >0.4 mmol l−1 min−1 (>7.2 mg dl−1 min−1). We also report post hoc calculations of the probabilities of benefit—that is, different from zero—both separately and jointly. Prespecified comparisons were for iTRE versus CR and for iTRE + CR versus the standard care. In addition, we present comparisons of 95% central prior and posterior ellipses. These are the two-dimensional equivalent of 95% CI. The analyses were in individuals with both HbA1c and postprandial glucose measures at month 6. Six individuals had HbA1c data but were missing postprandial glucose change data, and were excluded from this analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Anonymized data from this study are available on request from the corresponding author for 36 months from date of publication with a full research plan for academic use only. The data are not publicly available as they contain information that could compromise research participant consent.
Code availability
No unique software or computational code was created for this study.
References
Diabetes Prevention Program Research Group et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374, 1677–1686 (2009).
Uusitupa, M. et al. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients 11, 2611 (2019).
Evert, A. B. et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 42, 731–754 (2019).
Patikorn, C. et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw. Open 4, e2139558 (2021).
Harvie, M. et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 110, 1534–1547 (2013).
Harvie, M. N. et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J. Obes. (Lond.) 35, 714–727 (2011).
Di Francesco, A. et al. A time to fast. Science 362, 770–775 (2018).
Lawson, C. A. et al. Animal models of GM2 gangliosidosis: utility and limitations. Appl. Clin. Genet. 9, 111–120 (2016).
Tiribuzi, R. et al. Lysosomal β-galactosidase and β-hexosaminidase activities correlate with clinical stages of dementia associated with Alzheimer’s disease and type 2 diabetes mellitus. J. Alzheimers Dis. 24, 785–797 (2011).
Hultberg, B. et al. beta-Hexosaminidase isoenzymes A and B in middle-aged and elderly subjects: determinants of plasma levels and relation to vascular disease. Ann. Clin. Biochem. 33, 432–437 (1996).
Kim, H. K. et al. Renal tubular damage marker, urinary N-acetyl-β-d-glucosaminidase, as a predictive marker of hepatic fibrosis in type 2 diabetes mellitus. Diabetes Metab. J. 46, 104–116 (2022).
Montgomery, M. K. et al. Hexosaminidase A (HEXA) regulates hepatic sphingolipid and lipoprotein metabolism in mice. FASEB J. 35, e22046 (2021).
Teong, X. T. et al. Evidence gaps and potential roles of intermittent fasting in the prevention of chronic diseases. Exp. Gerontol. 153, 111506 (2021).
Acosta-Rodriguez, V. et al. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 376, 1192–1202 (2022).
Pak, H. H. et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat. Metab. 3, 1327–1341 (2021).
Froy, O. et al. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech. Ageing Dev. 130, 154–160 (2009).
Regmi, P. et al. Time-restricted eating: benefits, mechanisms, and challenges in translation. iScience 23, 101161 (2020).
Liu, D. et al. Calorie restriction with or without time-restricted eating in weight loss. N. Engl. J. Med. 386, 1495–1504 (2022).
Thomas, E. A. et al. Early time-restricted eating compared with daily caloric restriction: a randomized trial in adults with obesity. Obesity (Silver Spring) 30, 1027–1038 (2022).
Jamshed, H. et al. Effectiveness of early time-restricted eating for weight loss, fat loss, and cardiometabolic health in adults with obesity: a randomized clinical trial. JAMA Intern. Med. 182, 953–962 (2022).
Anton, S. D. et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring) 26, 254–268 (2018).
Trepanowski, J. F. et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern. Med. 177, 930–938 (2017).
Sundfor, T. M. et al. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 28, 698–706 (2018).
Shankar, S. S. et al. Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: results from the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care 39, 1602–1613 (2016).
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Lind, M. et al. The association between HbA1c, fasting glucose, 1-hour glucose and 2-hour glucose during an oral glucose tolerance test and cardiovascular disease in individuals with elevated risk for diabetes. PLoS ONE 9, e109506 (2014).
Cavalot, F. et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J. Clin. Endocrinol. Metab. 91, 813–819 (2006).
Antoni, R. et al. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br. J. Nutr. 119, 507–516 (2018).
Templeman, I. et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 13, eabd8034 (2021).
Gao, Y. et al. Effects of intermittent (5:2) or continuous energy restriction on basal and postprandial metabolism: a randomised study in normal-weight, young participants. Eur. J. Clin. Nutr. 76, 65–71 (2022).
Jones, R. et al. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am. J. Clin. Nutr. 112, 1015–1028 (2020).
Karpe, F. et al. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60, 2441–2449 (2011).
Phillips, D. I. W. et al. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet. Med. 11, 286–292 (1994).
Horowitz, M. et al. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 36, 857–862 (1993).
Hutchison, A. T. et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring) 27, 724–732 (2019).
Hemmingsen, B. et al. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 12, CD003054 (2017).
Dashti, H. S. et al. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 113, 154–161 (2020).
Jamshed, H. et al. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 11, 1234 (2019).
Sutton, E. F. et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221 (2018).
Vujović, N. et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 34, 1486–1498 (2022).
Wehrens, S. M. T. et al. Meal timing regulates the human circadian system. Curr. Biol. 27, 1768–1775 (2017).
Van Namen, M. et al. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: a systematic review and meta-analysis. Metabolism 101, 153988 (2019).
Keirns, B. H. et al. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J. Nutr. Sci. 10, e75 (2021).
Hultberg, B. et al. Pattern of serum beta-hexosaminidase in liver cirrhosis. Scand. J. Gastroenterol. 18, 877–880 (1983).
Poon, P. Y. et al. Plasma N-acetyl-beta-d-glucosaminidase activities and glycaemia in diabetes mellitus. Diabetologia 24, 433–436 (1983).
Whiting, P. H. et al. Serum and urine N-acetyl-beta-d-glucosaminidase in diabetics on diagnosis and subsequent treatment, and stable insulin dependent diabetics. Clin. Chim. Acta 92, 459–463 (1979).
Lipina, C. et al. Ganglioside GM3 as a gatekeeper of obesity-associated insulin resistance: evidence and mechanisms. FEBS Lett. 589, 3221–3227 (2015).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation 129, S102–S138 (2014).
Teong, X. T. et al. Rationale and protocol for a randomized controlled trial comparing daily calorie restriction versus intermittent fasting to improve glycaemia in individuals at increased risk of developing type 2 diabetes. Obes. Res Clin. Pract. 14, 176–183 (2020).
Teong, X. T. et al. An update to the study protocol for a randomized controlled trial comparing daily calorie restriction versus intermittent fasting to improve glycaemia in individuals at increased risk of developing type 2 diabetes. Obes. Res Clin. Pract. 15, 306 (2021).
Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (The National Academies Press, 2005).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Leaback, D. H. & Walker, P. G. Studies on glucosaminidase. 4. fluorimetric assay. N.-acetyl-beta-glucosaminidase. Biochem J. 78, 151–156 (1961).
Whyte, L. S. et al. Lysosomal gene Hexb displays haploinsufficiency in a knock-in mouse model of Alzheimer’s disease. IBRO Neurosci. Rep. 12, 131–141 (2022).
Acknowledgements
This work was supported by the National Health and Medical Research Council Project Grant (APP1143092). X.T.T. was supported by an Australian Government Research Training Program Scholarship from The University of Adelaide. This work was supported by a Diabetes Australia Research Program Grant (Y21G-SART) awarded to T.J.S., J.B. and L.K.H. The funder had no role in the design of this study and the interpretation of the study results. We thank all the trial participants.
Author information
Authors and Affiliations
Contributions
L.K.H., A.T.H., C.F.-B., A.D.V. and G.A.W. designed the study. A.D.V. wrote the statistical analysis plan, performed randomization and statistical analysis. X.T.T., K.L., B.L., L.Z. and A.T.H. collected the blood samples. G.A.W. provided clinical support and supervision. X.T.T., K.L., A.D.V. and L.K.H. analyzed the data. J.B., K.J.H. and T.J.S. measured the β-hexosaminidase activity. All authors critically revised the draft and approved the final manuscript. L.K.H. had full access to the data and had primary responsibility for the final publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Jonathan Little, Krista Varady, Luke Ouma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparisons of (a) iTRE vs CR, and (b) iTRE + CR vs SC for joint change in postprandial glucose (mg/dL/min) and HbA1c (%).
Points indicate prior (blue), posterior (red) and observed means (black). The ellipses indicate 95% central prior (blue) and posterior (red) probabilities. We note that our prior belief was for a correlation of 0.7 in change of the two outcomes, which was not observed. iTRE, intermittent time-restricted diet at 70% of calculated energy requirements; CR, calorie restriction diet at 70% of calculated daily energy requirements; SC: standard care diet.
Extended Data Fig. 2 Weight loss (kg) relative to baseline.
Presented are means and 95% CIs (calculated as \(\overline x \pm 1.96\,{{{\mathrm{SEM}}}}\)) of the non-fasting weight change by group during each face to face check-in visit. Treatment group trajectories were compared using linear mixed effects regression assuming piecewise linear effects assumed for the interventions over two time periods: month 0–6 and month 7–18, and both random intercepts and slopes for individuals. iTRE, intermittent time-restricted diet at 70% of calculated energy requirements; CR, calorie restriction diet at 70% of calculated daily energy requirements; SC: standard care diet.
Extended Data Fig. 3 Weight loss percentage from baseline to month 6 in iTRE (a), CR (b), SC (c).
iTRE, intermittent time-restricted diet at 70% of calculated energy requirements; CR, calorie restriction diet at 70% of calculated daily energy requirements; SC: standard care diet.
Supplementary information
Supplementary Information
Supplementary Tables 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teong, X.T., Liu, K., Vincent, A.D. et al. Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: a randomized controlled trial. Nat Med 29, 963–972 (2023). https://doi.org/10.1038/s41591-023-02287-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02287-7
This article is cited by
-
Early time-restricted carbohydrate consumption vs conventional dieting in type 2 diabetes: a randomised controlled trial
Diabetologia (2024)
-
Food as medicine: translating the evidence
Nature Medicine (2023)
-
Intermittent fasting and Alzheimer's disease—Targeting ketone bodies as a potential strategy for brain energy rescue
Metabolic Brain Disease (2023)