Abstract
Background
Despite known sex-based differences in cardiovascular aging, differences in aging biology are poorly understood. We hypothesize that circulating metabolites studied cross-sectionally with cardiac aging may be associated with cardiovascular changes that distinguish cardiac aging in women.
Methods
A population-based cohort of community men and women without cardiovascular disease from Singapore underwent detailed clinical and echocardiography examinations. Cross-sectional associations between cardiac functional characteristics and metabolomics profiles were examined.
Results
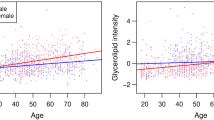
Five hundred sixty-seven adults (48.9% women) participated. Women were younger (72 ± 4.4 years vs 73 ± 4.3 years, p = 0.022), had lower diastolic blood pressures (71 ± 11.0 mmHg vs 76 ± 11.2 mmHg, p < 0.0001, and less likely to have diabetes mellitus (18.0% vs 27.6%, p = 0.013) and smoking (3.8% vs 34.5%, p < 0.001). Body mass indices were similar (24 ± 3.8 kg/m2 vs 24 ± 3.4 kg/m2, p = 0.29), but women had smaller waist circumferences (81 ± 10.1 cm vs 85 ± 9.2 cm, p < 0.001). Women had a significantly higher E/e′ ratios (10.9 ± 3.4 vs 9.9 ± 3.3, p = 0.007) and mitral A peak (0.86 ± 0.2 m/s vs 0.79 ± 0.2 m/s, p < 0.001) than men. Among women, lower E/e′ ratio was associated with higher levels of C16 (OR 1.019, 95%CI 1.002–1.036, p = 0.029), C16:1 (OR 1.06, 95%CI 1.006–1.118, p = 0.028), serine (OR 1.019, 95%CI 1.002–1.036, p = 0.025), and histidine (OR 1.045, 95%CI 1.013–1.078, p = 0.006). Lower mitral A peak was associated with higher levels of histidine (OR 1.039, 95%CI 1.009–1.070, p = 0.011), isoleucine (OR 1.013, 95%CI 1.004–1.021, p = 0.004), and C20 (OR 1.341, 95%CI 1.067–1.684, p = 0.012).
Conclusion
Impairments in diastolic functions were more frequent among older women compared to men, despite lower prevalence of vascular risk factors and preserved cardiac structure. Cardiac aging in women correlated with metabolites involved in fatty acid oxidation and tricyclic acid cycle fuelling.



Similar content being viewed by others
Data availability statement
The data underlying this article cannot be shared publicly due to institutional restrictions. The data will be shared on reasonable request to the corresponding author.
Abbreviations
- A′:
-
Peak tissue velocity of the mitral annulus in late diastole
- AO:
-
Ascending aorta diameter
- CI:
-
Confidence interval
- DT:
-
Deceleration time
- E′:
-
Peak tissue velocity of the mitral annulus in early diastole
- E/A ratio:
-
Ratio of mitral E peak to mitral A peak
- E/e′ ratio:
-
Ratio of mitral E peak to e'
- IVRT:
-
Isovolumetric relaxation time
- IVSd:
-
Interventricular septum thickness in diastole
- IVSs:
-
Interventricular septum thickness in systole
- LA:
-
Left atrium
- LAVI:
-
Left atrium volume index
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- LVIDd:
-
Left ventricle internal diameter in diastole
- LVIDs:
-
Left ventricle internal diameter in systole
- LVMI:
-
Left ventricle mass index
- LVOT:
-
Left ventricle outflow tract
- LVPWd:
-
Left ventricle posterior wall thickness in diastole
- LVPWs:
-
Left ventricle posterior wall thickness in systole
- Mitral A peak:
-
Peak transmitral velocity in late diastole
- Mitral E peak:
-
Peak transmitral velocity in early diastole
- PASP:
-
Pulmonary artery systolic pressure
- S′:
-
Peak tissue velocity of the mitral annulus in systole
References
Merz AA, Cheng S (2016) Sex differences in cardiovascular ageing. Heart 102(11):825. https://doi.org/10.1136/heartjnl-2015-308769
Hayward CS, Kelly RP, Collins P (2000) The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res 46(1):28–49. https://doi.org/10.1016/s0008-6363(00)00005-5
Swaraj S, Kozor R, Arnott C, Di Bartolo BA, Figtree GA (2021) Heart failure with reduced ejection fraction-does sex matter? Curr Heart Fail Rep 18(6):345–352. https://doi.org/10.1007/s11897-021-00533-y
Oktay AA, Rich JD, Shah SJ (2013) The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 10(4):401–410. https://doi.org/10.1007/s11897-013-0155-7
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA (2005) Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112(15):2254–2262. https://doi.org/10.1161/CIRCULATIONAHA.105.541078
Pauls SD et al (2021) Impact of age, menopause, and obesity on oxylipins linked to vascular health. Arterioscler Thromb Vasc Biol 41(2):883–897. https://doi.org/10.1161/ATVBAHA.120.315133
Huang T et al (2019) Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women. Int J Epidemiol 48(4):1262–1274. https://doi.org/10.1093/ije/dyy234
Campesi I et al (2016) Ageing/menopausal status in healthy women and ageing in healthy men differently affect cardiometabolic parameters. Int J Med Sci 13(2):124–132. https://doi.org/10.7150/ijms.14163
Auro K et al (2014) A metabolic view on menopause and ageing. Nat Commun 5(1):4708. https://doi.org/10.1038/ncomms5708
Shah SH, Newgard CB (2015) Integrated metabolomics and genomics. Circ Cardiovasc Genet 8(2):410–419. https://doi.org/10.1161/CIRCGENETICS.114.000223
Fujimoto N et al (2012) Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol 590(8):1871–1880. https://doi.org/10.1113/jphysiol.2011.218271
Shah SJ et al (2020) Research priorities for heart failure with preserved ejection fraction. Circulation 141(12):1001–1026. https://doi.org/10.1161/CIRCULATIONAHA.119.041886
Vogel MW, Slusser JP, Hodge DO, Chen HH (2012) The natural history of preclinical diastolic dysfunction: a population-based study. Circ Heart Fail 5(2):144–151. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959668
Strait JB, Lakatta EG (2012) Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 8(1):143–164. https://doi.org/10.1016/j.hfc.2011.08.011
From AM, Scott CG, Chen HH (2010) The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 55(4):300–305. https://doi.org/10.1016/j.jacc.2009.12.003
Kovalik JP et al (2021) Amino acid differences between diabetic older adults and non-diabetic older adults and their associations with cardiovascular function. J Mol Cell Cardiol 158:63–71. https://doi.org/10.1016/j.yjmcc.2021.05.009
Hankin JH et al (2001) Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 39(2):187–195. https://doi.org/10.1207/S15327914nc392_5
Denchev SV, Simova II, Matveev MG (2007) Evaluation of the SCHILLER BR-102 plus noninvasive ambulatory blood pressure monitor according to the International Protocol introduced by the Working Group on Blood Pressure Monitoring of the European Society of Hypertension. Blood Press Monit 12(5):329–333. https://doi.org/10.1097/MBP.0b013e32813fa39e
Nes BM, Janszky I, Vatten LJ, Nilsen TI, Aspenes ST, Wisløff U (2011) Estimating V·O 2peak from a nonexercise prediction model: the HUNT Study, Norway. Med Sci Sports Exerc 43(11):2024–2030. https://doi.org/10.1249/MSS.0b013e31821d3f6f
Nes BM, Vatten LJ, Nauman J, Janszky I, Wisløff U (2014) A simple nonexercise model of cardiorespiratory fitness predicts long-term mortality. Med Sci Sports Exerc 46(6):1159–1165. https://doi.org/10.1249/mss.0000000000000219
Koh AS et al (2018) Metabolomic correlates of aerobic capacity among elderly adults. Clin Cardiol 41(10):1300–1307. https://doi.org/10.1002/clc.23016
Lang RM et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39. https://doi.org/10.1016/j.echo.2014.10.003
Gao F et al (2021) Exacerbation of cardiovascular ageing by diabetes mellitus and its associations with acyl-carnitines. Aging 13(11):14785–14805. https://doi.org/10.18632/aging.203144
Upadhya B, Kitzman DW (2017) Heart failure with preserved ejection fraction in older adults. Heart Fail Clin 13(3):485–502. https://doi.org/10.1016/j.hfc.2017.02.005
Kobak KA, Zarzycka W, Chiao YA (2022) Age and sex differences in heart failure with preserved ejection fraction. Front Aging. https://doi.org/10.3389/fragi.2022.811436
Paulus WJ et al (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550
Eaton CB et al (2016) Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002883
Hunter WG et al (2016) Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. https://doi.org/10.1161/jaha.115.003190
Cheng ML et al (2015) Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol 65(15):1509–1520. https://doi.org/10.1016/j.jacc.2015.02.018
Koh AS et al (2018) Dissecting clinical and metabolomics associations of left atrial phasic function by cardiac magnetic resonance feature tracking. Sci Rep 8(1):8138–8138. https://doi.org/10.1038/s41598-018-26456-8
Rinaldo P, Cowan TM, Matern D (2008) Acylcarnitine profile analysis. Genet Med 10(2):151–156. https://doi.org/10.1097/GIM.0b013e3181614289
Makrecka-Kuka M et al (2017) Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci Rep 7(1):17528. https://doi.org/10.1038/s41598-017-17797-x
Molina AJ et al (2016) Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC Heart Fail 4(8):636–645. https://doi.org/10.1016/j.jchf.2016.03.011
Rutkowsky JM et al (2014) Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab 306(12):E1378–E1387. https://doi.org/10.1152/ajpendo.00656.2013
Koh A et al (2017) Metabolomic profile of arterial stiffness in aged adults. Diab Vasc Dis Res 15:147916411773362. https://doi.org/10.1177/1479164117733627
van der Zwaard S et al (2016) Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J Appl Physiol (1985) 121(3):636–645. https://doi.org/10.1152/japplphysiol.00355.2016
Betik AC, Hepple RT (2008) Determinants of VO2 max decline with aging: an integrated perspective. Appl Physiol Nutr Metab 33(1):130–140. https://doi.org/10.1139/h07-174
Magkos F et al (2013) Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes 62(8):2757–2761. https://doi.org/10.2337/db13-0185
Chng CL et al (2016) Physiological and metabolic changes during the transition from hyperthyroidism to euthyroidism in graves’ disease. Thyroid 26(10):1422–1430. https://doi.org/10.1089/thy.2015.0602
Bao XR et al (2016) Mitochondrial dysfunction remodels one-carbon metabolism in human cells. Elife. https://doi.org/10.7554/eLife.10575
Razavi AC et al (2020) Novel findings from a metabolomics study of left ventricular diastolic function: the Bogalusa heart study. J Am Heart Assoc 9(3):e015118. https://doi.org/10.1161/JAHA.119.015118
Previtali M, Chieffo E, Ferrario M, Klersy C (2012) Is mitral E/E′ ratio a reliable predictor of left ventricular diastolic pressures in patients without heart failure? European Heart Journal - Cardiovascular Imaging 13(7):588–595. https://doi.org/10.1093/ejechocard/jer286
Sharp AS et al (2010) Tissue Doppler E/E’ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J 31(6):747–752. https://doi.org/10.1093/eurheartj/ehp498
Kim HL et al (2013) The association between arterial stiffness and left ventricular filling pressure in an apparently healthy Korean population. Cardiovasc Ultrasound 11(1):2. https://doi.org/10.1186/1476-7120-11-2
H-L Kim et al (2017) Association between arterial stiffness and left ventricular diastolic function in relation to gender and age. Medicine 96(1) [Online]. https://journals.lww.com/md-journal/Fulltext/2017/01060/Association_between_arterial_stiffness_and_left.47.aspx.
Wu J, Yu SY, Wo D, Zhao MM, Zhang LJ, Li J (2016) Risks and predictors of mild diastolic dysfunction among middle-aged and aged women: a population-based cohort study. J Hum Hypertens 30(5):335–340. https://doi.org/10.1038/jhh.2015.85
Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L (2014) Role of estrogen in diastolic dysfunction. American Journal of Physiology-Heart and Circulatory Physiology 306(5):H628–H640. https://doi.org/10.1152/ajpheart.00859.2013
Mathew S et al (2014) Metabolomics of Ramadan fasting: an opportunity for the controlled study of physiological responses to food intake. J Transl Med 12:161. https://doi.org/10.1186/1479-5876-12-161
Carayol M et al (2015) Reliability of serum metabolites over a two-year period: a targeted metabolomic approach in fasting and non-fasting samples from EPIC. PLoS ONE 10(8):e0135437. https://doi.org/10.1371/journal.pone.0135437
Hancox RJ, Landhuis CE (2011) Correlation between measures of insulin resistance in fasting and non-fasting blood. Diabetol Metab Syndr 3(1):23. https://doi.org/10.1186/1758-5996-3-23
Wallace TM, Matthews DR (2002) The assessment of insulin resistance in man. Diabet Med. https://doi.org/10.1046/j.1464-5491.2002.00745.x
Acknowledgements
We thank staff and collaborators from the imaging and research laboratories for participating in the conduct of the study.
Funding
The Cardiac Aging Study has received funding support from the National Medical Research Council of Singapore (MOH-000153, HLCA21Jan-0052), Hong Leong Foundation, Duke-NUS Medical School, Estate of Tan Sri Khoo Teck Puat and Singhealth Foundation. Woon-Puay Koh is supported by the National Medical Research Council, Singapore (MOH-CSASI19nov-0001). The funders had no role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
ASK, J-PK, LLYT, SHE, R-ST, W-PK contributed to the conception and design of the work. FG, WHN, ASK, JC, KVC, LSL contributed to the acquisition, analysis and interpretation of the data. The first draft of the manuscript was written by JSH and JJW. All authors have participated in reviewing and/or revising the manuscript and have approved its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ho, J.S., Wong, J.J., Gao, F. et al. Adverse cardiovascular and metabolic perturbations among older women: ‘fat-craving’ hearts. Clin Res Cardiol 112, 1555–1567 (2023). https://doi.org/10.1007/s00392-023-02156-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02156-w